All Photos(1)
About This Item
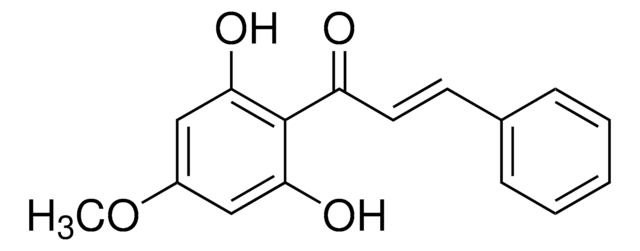
Linear Formula:
CH3OC6H4CH=CHCOC6H2(OCH3)2OH
CAS Number:
Molecular Weight:
314.33
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
112-116 °C (lit.)
functional group
ketone
SMILES string
COc1ccc(\C=C\C(=O)c2c(O)cc(OC)cc2OC)cc1
InChI
1S/C18H18O5/c1-21-13-7-4-12(5-8-13)6-9-15(19)18-16(20)10-14(22-2)11-17(18)23-3/h4-11,20H,1-3H3/b9-6+
InChI key
CGIBCVBDFUTMPT-RMKNXTFCSA-N
Related Categories
Application
2′-Hydroxy-4,4′,6′-trimethoxychalcone may be used to synthesize 2′,2”′-dihydroxy-4,4′,4′′,4”′,6′,6′′′-hexamethoxy[5′,5′′′]bichalcone and 3′-bromo-4,4′,6′-trimethoxy-2′-hydroxychalcone.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Regioselective bromination of organic substrates by tetrabutylammonium bromide promoted by V2O5-H2O2: An environmentally favorable synthetic protocol.
Bora U, et al.
Organic Letters, 2(3), 247-249 (2000)
Practical conversion of artemisinic acid in desoxyartemisinin.
Jung M, et al.
The Journal of Organic Chemistry, 51(26), 5417-5419 (1986)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service