About This Item
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.557 (lit.)
bp
107 °C/3 mmHg (lit.)
density
1.224 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
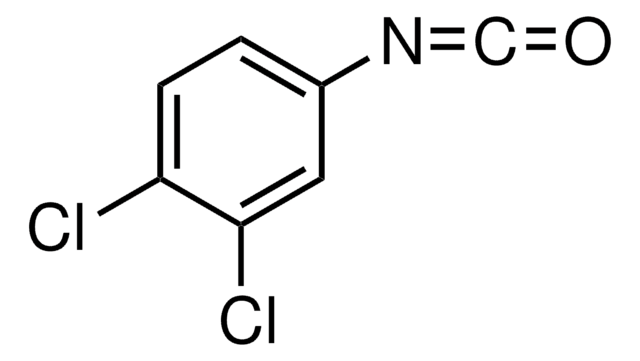
Cc1ccc(cc1Cl)N=C=O
InChI
1S/C8H6ClNO/c1-6-2-3-7(10-5-11)4-8(6)9/h2-4H,1H3
InChI key
UKTKKMZDESVUEE-UHFFFAOYSA-N
Related Categories
General description
Application
- 1-(1-(1-adamantyl)methyl)-3-(3-chloro-4-methylphenyl)urea
- 1-(3-chloro-4-methylphenyl)-3-heptylurea
- 1-(3-chloro-4-methylphenyl)-3-cyclooctylurea
- 1-(3-chloro-4-methylphenyl)-3-(3-fluorobenzyl)urea
- 1-(3-chloro-4-methylphenyl)-3-(4-phenylbutan-2-yl)urea
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service