All Photos(1)
About This Item
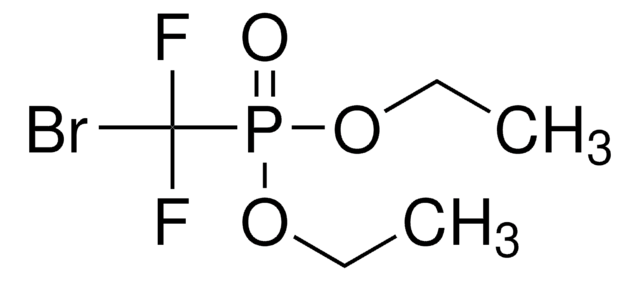
Linear Formula:
CH3O(CH2)3CO2CH3
CAS Number:
Molecular Weight:
132.16
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.408 (lit.)
bp
162-164 °C/767 mmHg (lit.)
density
0.969 g/mL at 25 °C (lit.)
functional group
ester
ether
SMILES string
COCCCC(=O)OC
InChI
1S/C6H12O3/c1-8-5-3-4-6(7)9-2/h3-5H2,1-2H3
InChI key
VHDGWXQBVWAMJA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Hazard Classifications
Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
129.2 °F - closed cup
Flash Point(C)
54 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Carlos Alemán et al.
The Journal of organic chemistry, 70(19), 7731-7736 (2005-09-10)
[structure: see text] A systematic conformational study of omega-methoxy methyl esters, CH(3)O-(CH2)n-COO-CH3 with n = 3 and 4, has been performed using quantum mechanical calculations at the MP2 level. Calculations have been carried out in both gas phase and chloroform
Maria Cammenberg et al.
Chembiochem : a European journal of chemical biology, 7(11), 1745-1749 (2006-09-23)
One of the commercial methods for preparing enantiopure amines is lipase-catalyzed kinetic resolution, although lipases catalyze aminolysis with only low activity. Interestingly, in 1997 Balkenhohl et al. used ethyl methoxyacetate instead of ethyl butyrate as an acylation reagent for the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service