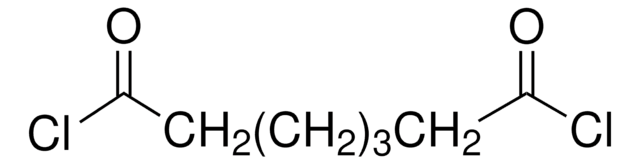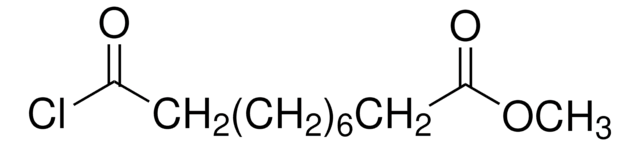385565
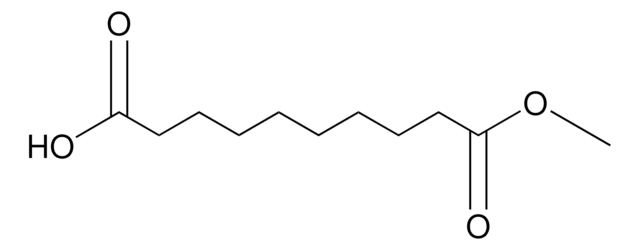
Methyl 8-chloro-8-oxooctanoate
96%
Synonym(s):
Methyl suberyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClCO(CH2)6CO2CH3
CAS Number:
Molecular Weight:
206.67
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.45 (lit.)
density
1.456 g/mL at 25 °C (lit.)
functional group
acyl chloride
ester
SMILES string
COC(=O)CCCCCCC(Cl)=O
InChI
1S/C9H15ClO3/c1-13-9(12)7-5-3-2-4-6-8(10)11/h2-7H2,1H3
InChI key
RKUPOLBFJIEWBZ-UHFFFAOYSA-N
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and properties of PI polyamide?SAHA conjugate.
Ohtsuki et al.
Tetrahedron Letters, 50(52), 7288-7292 (2009)
Laura Forster et al.
Analytical and bioanalytical chemistry, 394(6), 1679-1685 (2009-06-02)
A fluorescent assay for the evaluation of inhibitors of fatty acid amide hydrolase (FAAH) is described. Microsomes from rat brain served as enzyme source. N-(2-Hydroxyethyl)-4-pyren-1-ylbutanamide was designed and synthesized as novel fluorogenic substrate. For substrate solubilization, Triton X-100 was employed.
John Spencer et al.
ACS medicinal chemistry letters, 2(5), 358-362 (2011-05-17)
N(1)-Hydroxy-N(8)-ferrocenyloctanediamide, JAHA (7), an organometallic analogue of SAHA containing a ferrocenyl group as a phenyl bioisostere, displays nanomolar inhibition of class I HDACs, excellent selectivity over class IIa HDACs, and anticancer action in intact cells (IC(50) = 2.4 μM, MCF7
A cyclodextrin-capped histone deacetylase inhibitor.
Amin et al.
Biochemical Medicine, 23(11), 3346-3348 (2013)
Synthesis of 1-Aminocyclopropyl-Pentanoic and-Heptanoic Acid Derivatives.
Gensini et al.
Letters in Organic Chemistry, 5(5), 328-331 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service