286958
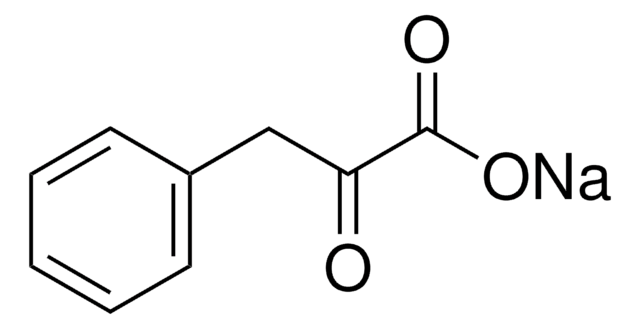
Phenylpyruvic acid
98%
Synonym(s):
2-Oxo-3-phenylpropanoic acid, 2-Oxo-3-phenylpropionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5CH2COCOOH
CAS Number:
Molecular Weight:
164.16
Beilstein:
2207312
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
150-154 °C (lit.)
functional group
carboxylic acid
ketone
phenyl
storage temp.
−20°C
SMILES string
OC(=O)C(=O)Cc1ccccc1
InChI
1S/C9H8O3/c10-8(9(11)12)6-7-4-2-1-3-5-7/h1-5H,6H2,(H,11,12)
InChI key
BTNMPGBKDVTSJY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Phenylpyruvic acid reduces glucose-6-phosphate dehydrogenase activity without pre-incubation.
Application
Phenylpyruvic acid was used in the synthesis of 3-phenyllactic acid (PLA) by lactate dehydrogenase.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Shuhuai Yu et al.
Biotechnology letters, 36(3), 627-631 (2013-11-20)
3-Phenyllactic acid (PLA) is an antimicrobial compound with broad and effective antimicrobial activity against both bacteria and fungi. Enzymatic production of PLA can be carried out from phenylpyruvic acid by lactate dehydrogenase (LDH); however, the enzymatic reaction is accompanied by
Taiki Fujii et al.
Biochimica et biophysica acta, 1814(12), 1669-1676 (2011-06-16)
We discovered the phenyllactate (PLA)-producing fungal strain Wickerhamia fluorescens TK1 and purified phenylpyruvate reductase (PPR) from fungal cell-free extracts. The PPR used both NADPH and NADH as cofactors with more preference for the former. The enzyme reaction as well as
Andrea Pereira Rosa et al.
Cellular and molecular neurobiology, 32(7), 1113-1118 (2012-04-06)
Phenylketonuria is a recessive autosomal disorder that is caused by a deficiency in the activity of phenylalanine-4-hydroxylase, which converts phenylalanine to tyrosine, leading to the accumulation of phenylalanine and its metabolites phenyllactic acid, phenylacetic acid, and phenylpyruvic acid in the
A Hargreaves et al.
Journal of photochemistry and photobiology. B, Biology, 89(2-3), 110-116 (2007-11-06)
Ultraviolet A (UVA) light (315-400 nm) is ubiquitously found in our environment and constitutes about 95% of the total solar UV; all UVC and most UVB being absorbed by the stratospheric ozone layer. Compared with UVB and C, UVA does
Tapan Kanti Paine et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 13(21), 6073-6081 (2007-05-01)
Iron(II)-phenylpyruvate complexes of tetradentate tris(6-methyl-2-pyridylmethyl)amine (6-Me3-TPA) and tridentate benzyl bis(2-quinolinylmethyl)amine (Bn-BQA) were prepared to gain insight into C-C bond cleavage catalyzed by dioxygenase enzymes. The complexes we have prepared and characterized are [Fe(6-Me3-tpa)(prv)][BPh4] (1), [Fe2(6-Me3-tpa)2(pp)][(BPh4)2] (2), and [Fe2(6-Me3-tpa)2(2'-NO2-pp)][(BPh4)2] (3), [Fe(6-Me3-tpa)(pp-Me)][BPh4]
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service