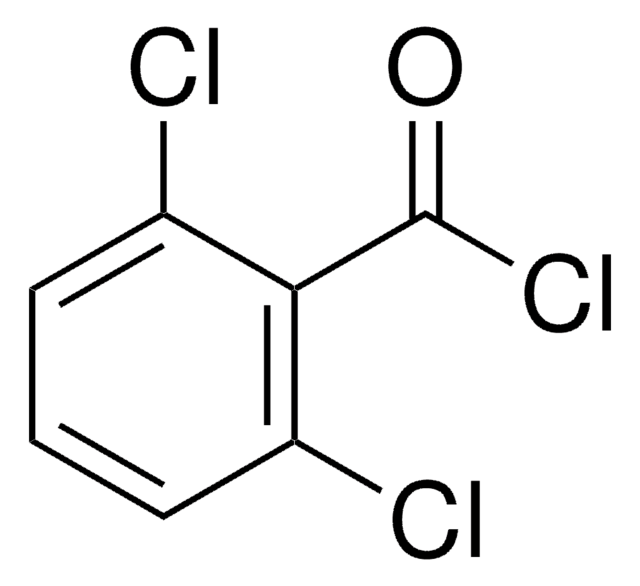
259179
3,4-Dichlorobenzyl chloride
97%
Synonym(s):
α,3,4-Trichlorotoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Cl2C6H3CH2Cl
CAS Number:
Molecular Weight:
195.47
Beilstein:
386644
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.577 (lit.)
bp
122-124 °C/14 mmHg (lit.)
density
1.411 g/mL at 25 °C (lit.)
functional group
chloro
SMILES string
ClCc1ccc(Cl)c(Cl)c1
InChI
1S/C7H5Cl3/c8-4-5-1-2-6(9)7(10)3-5/h1-3H,4H2
InChI key
YZIFVWOCPGPNHB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3,4-Dichlorobenzyl chloride has been used:
- in Friedel-Crafts synthesis of 4-(3,4-dichlorophenyl)-3,4-dihydro-1(2H)-naphthalenone, a key intermediate for the synthesis of sertraline
- as alkylating agent in synthesis of poly(ether ketone)s having pendant sulfonic acid groups
- in preparation of 5,6-dichloro-2-(3,4-dichlorobenzylthio)benzimidazole
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Syntheses of branched poly (ether ketone) s with pendant functional groups based on 1, 1, 1-tris (4-hydroxyphenyl) ethane.
Fritsch D, et al.
Journal of Macromolecular Science, Part A: Pure and Applied Chemistry, 39(11), 1335-1347 (2002)
Friedel-Crafts synthesis of 4-(3, 4-dichlorophenyl)-3, 4-dihydro-1 (2H)-naphthalenone, a key intermediate in the preparation of the antidepressant sertraline.
Quallich GJ, et al.
The Journal of Organic Chemistry, 55(16), 4971-4973 (1990)
Zygmunt Kazimierczuk et al.
Acta biochimica Polonica, 49(1), 185-195 (2002-07-26)
Two series of benzimidazole derivatives were sythesised. The first one was based on 5,6-dinitrobenzimidazole, the second one comprises 2-thioalkyl- and thioaryl-substituted modified benzimidazoles. Antibacterial and antiprotozoal activity of the newly obtained compounds was studied. Some thioalkyl derivatives showed remarkable activity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service