137448
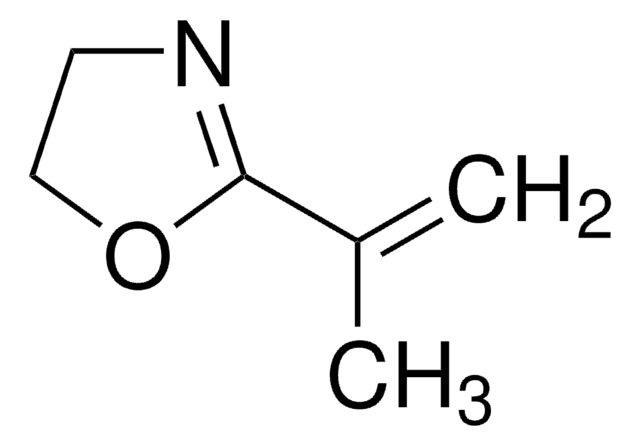
2-Methyl-2-oxazoline
98%
Synonym(s):
2-Methyloxazoline
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H7NO
CAS Number:
Molecular Weight:
85.10
Beilstein:
104227
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.434 (lit.)
bp
109.5-110.5 °C (lit.)
density
1.005 g/mL at 25 °C (lit.)
SMILES string
CC1=NCCO1
InChI
1S/C4H7NO/c1-4-5-2-3-6-4/h2-3H2,1H3
InChI key
GUXJXWKCUUWCLX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Methyl-2-oxazoline undergoes polymerization with 2-butyl-2-oxazoline to form poly(2-oxazoline) block copolymer. It undergoes cationic ring-opening polymerization with 2-(dec-9-enyl)-2-oxazoline to yield copoly(2-oxazoline)s.
Application
2-Methyl-2-oxazoline was used in cationic polymerization of a series of linear 2-alkyl-2-oxazolines under microwave irradiation.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
68.0 °F - closed cup
Flash Point(C)
20 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ke Mao et al.
Talanta, 168, 230-239 (2017-04-11)
In this work, a one-step coating procedure by a simple annealing protocol of poly (2-methyl-2-oxazoline)-random-glycidyl methacrylate (PMOXA-r-GMA) copolymer was used to yield covalent and cross-linked PMOXA-based antifouling coating on a fused-silica capillary inner surface, which was used to determine the
Ye Han Yan et al.
Journal of biomaterials science. Polymer edition, 31(4), 423-438 (2019-12-04)
In this work, the comb-like poly(2-methyl-2-oxazoline) copolymer, poly(2-aminoethyl methacrylate-random-poly(2-methyl-2-oxazoline) (PMOXA-r-AEMA, PMA) is synthesized, and the CuSO4/H2O2-triggered dopamine/PMA co-deposition process is investigated. Ellipsometry, water contact angle (WCA), and X-ray photoelectron spectroscopy (XPS) are used to characterize the thickness, hydrophilicity, and surface
Jing Tong et al.
Molecular pharmaceutics, 10(1), 360-377 (2012-11-21)
Superoxide dismutase 1 (SOD1) efficiently catalyzes dismutation of superoxide, but its poor delivery to the target sites in the body, such as brain, hinders its use as a therapeutic agent for superoxide-associated disorders. Here to enhance the delivery of SOD1
Denial Mahata et al.
Scientific reports, 7, 46412-46412 (2017-04-13)
Lignin, one of the most abundant renewable feedstock, is used to develop a biocompatible hydrogel as anti-infective ointment. A hydrophilic polyoxazoline chain is grafted through ring opening polymerization, possess homogeneous spherical nanoparticles of 10-15 nm. The copolymer was covalently modified with
Laetitia Korchia et al.
Soft matter, 13(25), 4507-4519 (2017-06-07)
A series of amphiphilic photo-responsive heterografted copolymers have been successfully synthesized. The random copolymers were composed of a methacrylate backbone, with various compositions of hydrophilic oligomeric 2-methyl-2-oxazoline side chains (OMOx) and hydrophobic long alkyl chains terminated by a coumarin moiety
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service