All Photos(1)
About This Item
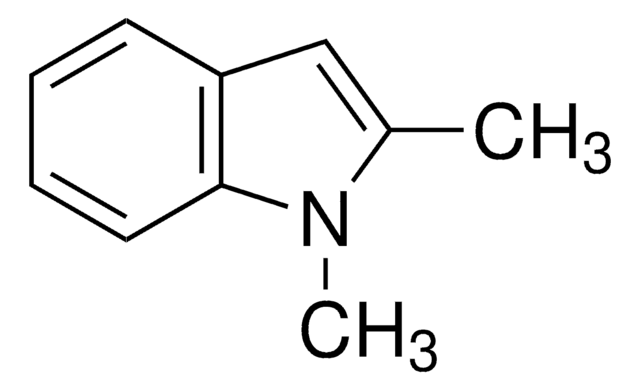
Empirical Formula (Hill Notation):
C10H11N
CAS Number:
Molecular Weight:
145.20
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97%
form
solid
bp
285 °C (lit.)
mp
105-107 °C (lit.)
SMILES string
Cc1[nH]c2ccccc2c1C
InChI
1S/C10H11N/c1-7-8(2)11-10-6-4-3-5-9(7)10/h3-6,11H,1-2H3
InChI key
PYFVEIDRTLBMHG-UHFFFAOYSA-N
Application
2,3-Dimethylindole has been used to study the mechanism of oxidation of 2,3-dimethylindole by peroxodisulphate and peroxomonosulphate anions to 2-methylindole-2-carbaldehyde. It has been used to study the behaviour of methylindoles in the agilent multimode ion source by atmospheric pressure chemical ionization mass spectrometry.
Reactant for preparation of:
Reactant for:
- Bis(indolyl)methane derivatives
- Potent opioid receptor agonists
- Photorefractive materials
- Prodrugs of the cyclin-dependent kinase (CDK) inhibitor Alsterpaullone
- Dopamine receptors 2/4 (D2/D4) antagonists
- Useful azaspirocyclic building blocks
Reactant for:
- Baylis-Hillman reactions
- Photosensitized Diels-Alder reactions
- Photoinduced electron transfer reactions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Direct evidence on the mechanism of the oxidation of 2, 3-dimethylindole by inorganic peroxo anions.
Balon M, et al.
The Journal of Organic Chemistry, 58(26), 7469-7473 (1993)
Formation of the Ions of Methylindoles in APCI Mass Spectrometry.
Liu DQ and Sun M.
ISRN Spectroscopy, 2012 (2012)
A I Novaira et al.
Journal of photochemistry and photobiology. B, Biology, 60(1), 25-31 (2001-06-02)
The quenching of anthracene fluorescence by indole, 1,2-dimethylindole (DMI), tryptophan (Trp) and indole 3-acetic acid (IAA) in palmitoyloleoylphosphatidylcholine (POPC) lipid bilayers was investigated. A very efficient quenching of the anthracene fluorescence in the lipid membrane is observed. Stern-Volmer plots are
Zhengyin Yan et al.
Analytical chemistry, 80(16), 6410-6422 (2008-07-23)
Constant neutral loss (CNL) and precursor ion (PI) scan have been widely used for the in vitro screening of glutathione conjugates derived from reactive metabolites, but these two methods are only applicable to triple quadrupole or hybrid triple quadrupole mass
Resolution of heterogeneous fluorescence from proteins and aromatic amino acids by phase-sensitive detection of fluorescence.
J R Lakowicz et al.
The Journal of biological chemistry, 256(12), 6348-6353 (1981-06-25)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service