46985
Fluorescein-5-thiosemicarbazide
suitable for fluorescence, ~80% (HPCE)
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
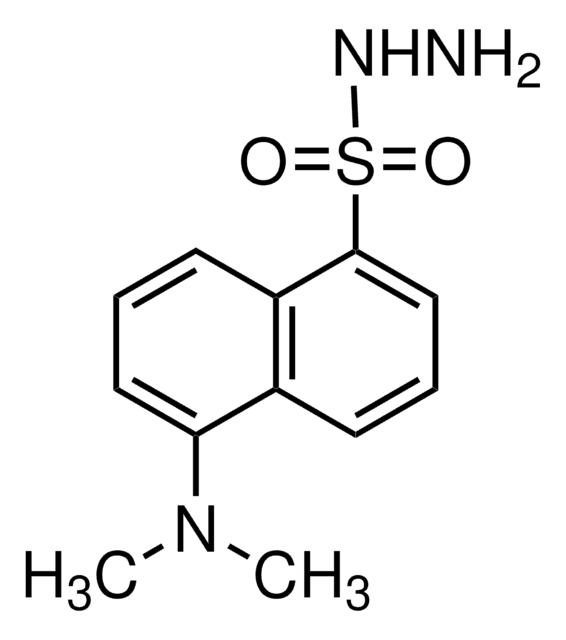
C21H15N3O5S
CAS Number:
Molecular Weight:
421.43
MDL number:
UNSPSC Code:
12352125
PubChem Substance ID:
NACRES:
NA.32
Recommended Products
Assay
~80% (HPCE)
form
solid
solubility
DMF: soluble
H2O: soluble
fluorescence
λex 492 nm; λem 516 nm in 0.1 M Tris pH 9.0
suitability
suitable for fluorescence
storage temp.
−20°C
SMILES string
NNC(=S)Nc1ccc(c(c1)C(O)=O)C2=C3C=CC(=O)C=C3Oc4cc(O)ccc24
InChI
1S/C21H15N3O5S/c22-24-21(30)23-10-1-4-13(16(7-10)20(27)28)19-14-5-2-11(25)8-17(14)29-18-9-12(26)3-6-15(18)19/h1-9,25H,22H2,(H,27,28)(H2,23,24,30)
InChI key
MEUCHQDLZYLNQY-UHFFFAOYSA-N
Related Categories
General description
Fluorescein-5-thiosemicarbazide, also known as FTC, is a fluorescent probe. The FTSC fluorescent spectra is like FITC, a preferred fluorescent tag for proteomics. The fluorescence spectral properties of FTSC-labeled vesicles obtained by reductive amination are fully compatible with the argon laser of fluorescent instruments.
Application
Fluorescein-5-thiosemicarbazide can be used as a fluorescent labeling reagent to label:
- Cell-surface functional groups (glycophorins) in the study of the effect of deoxygenation in the red blood cell membrane.
- Saccharides applicable in polysaccharide imaging in live cells.
- Chondroitin sulfate nanogels applicable in cell-specific drug delivery applications.
Features and Benefits
Fluorescein-5-thiosemicarbazide has the following benefits -
- It displays strong fluorescence property.
- Shows an intrinsic reactivity of the thiosemicarbazide group towards the aldehyde group.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Havé et al.
Plant biology (Stuttgart, Germany), 17(5), 973-979 (2015-02-17)
Leaf senescence is characterised by a massive degradation of proteins in order to recycle nitrogen to other parts of the plant, such as younger leaves or developing grain/seed. Protein degradation during leaf senescence is a highly regulated process and it
Ying Zhang et al.
Carbohydrate research, 346(14), 2156-2164 (2011-08-10)
A simple and efficient procedure for the fluorescent labeling of saccharides is a prerequisite step for imaging the transport of polysaccharides in living cells. We report a one-pot strategy for the fluorescent labeling of saccharides with fluorescein-5-thiosemicarbazide (FTSC), which introduces
Injectable hydrogel-incorporated cancer cell-specific cisplatin releasing nanogels for targeted drug delivery
Gil MS, et al.
Journal of Material Chemistry B: Materials for Biology and Medicine, 5(34), 7140-7152 (2017)
Deoxygenation affects fluorescence photobleaching recovery measurements of red cell membrane protein lateral mobility
Corbett JD, et al.
Biophysical Journal, 66(1), 25-30 (1994)
Yun Bai et al.
Nucleic acids research, 41(16), 7861-7874 (2013-06-21)
The 3' untranslated region (3'UTR) of hepatitis C virus (HCV) messenger RNA stimulates viral translation by an undetermined mechanism. We identified a high affinity interaction, conserved among different HCV genotypes, between the HCV 3'UTR and the host ribosome. The 3'UTR
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service