All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H11Cl2NO2
CAS Number:
Molecular Weight:
260.12
Beilstein:
4190275
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
SMILES string
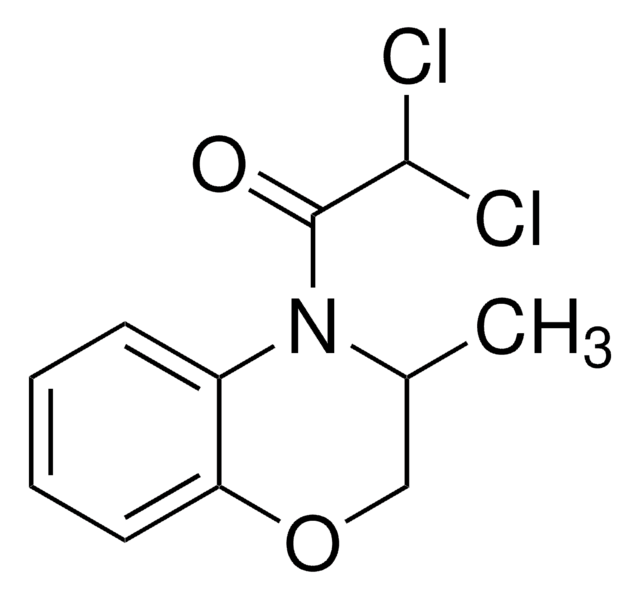
CC1COc2ccccc2N1C(=O)C(Cl)Cl
InChI
1S/C11H11Cl2NO2/c1-7-6-16-9-5-3-2-4-8(9)14(7)11(15)10(12)13/h2-5,7,10H,6H2,1H3
InChI key
PFJJMJDEVDLPNE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Benoxacor is a dichloroacetamide herbicide safener, used to protect corn against injury from metolachlor. Its role as a safener involves inducing the enzymatic mechanism of chloroacetanilide detoxification in plants.
Application
Benoxacor may be used as a reference standard in the determination of benoxacor in wheat and soil samples using gas chromatography (GC) and high performance liquid chromatography (HPLC).
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Legal Information
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 1 - Skin Sens. 1
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sihong Liu et al.
The Science of the total environment, 761, 143273-143273 (2020-11-17)
Benoxacor, a chiral herbicide safener for S-metolachlor, has been detected in streams. However, the potential risk this poses to aquatic ecosystems is not clear. This study used zebrafish (Danio rerio) embryos as a model to assess the enantioselective toxicity of
Derek Simonsen et al.
Chemico-biological interactions, 330, 109247-109247 (2020-09-01)
This study investigated the enantioselective metabolism of benoxacor, an ingredient of herbicide formulations, in microsomes or cytosol prepared from female or male rat livers. Benoxacor was incubated for ≤30 min with microsomes or cytosol, and its enantioselective depletion was measured using
C K Cottingham et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 53(11-12), 973-979 (1999-02-06)
The subcellular distribution of glutathione S-transferase (GST) activity extracted from shoots of 3-day-old etiolated seedlings of maize (Zea mays L., Northrup-King 9283 hybrid) and the induction of soluble and membrane-bound GST activity by the safener benoxacor, the herbicide metolachlor and
Purification and characterization of a glutathione S-transferase from benoxacor-treated maize (Zea mays).
Irzyk PG and Fuerst PE
Plant Physiology, 102(3), 803-810 (1993)
WSSA Abstracts: Meeting of the Weed Science Society of America, Volumes 25-32 (1985)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service