P1781
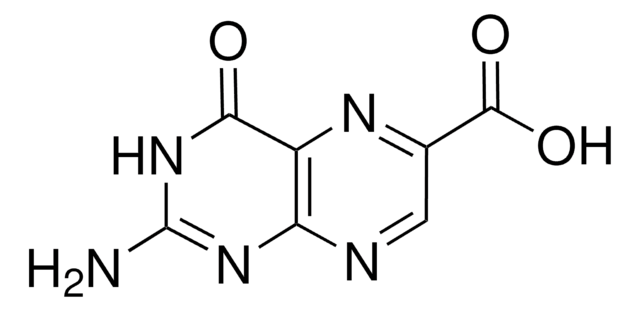
Pteroic acid
≥93%
Synonym(s):
4-{[(2-Amino-4-hydroxypteridin-6-yl)methyl]amino}benzoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H12N6O3
CAS Number:
Molecular Weight:
312.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥93%
form
powder
SMILES string
NC1=NC(=O)C2=NC(CNc3ccc(cc3)C(O)=O)=CNC2=N1
InChI
1S/C14H12N6O3/c15-14-19-11-10(12(21)20-14)18-9(6-17-11)5-16-8-3-1-7(2-4-8)13(22)23/h1-4,6,16H,5H2,(H,22,23)(H3,15,17,19,20,21)
InChI key
JOAQINSXLLMRCV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Pteroic acid can be used as a reactant to synthesize:
- N-trifluoroacetyl pteroic acid by reacting with trifluoroacetic anhydride via acylation.
- bis-Decyl chain derivative of pteroic acid through photocleavage reaction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Maciej Adamczyk et al.
Bioorganic & medicinal chemistry letters, 14(9), 2313-2317 (2004-04-15)
N(10)-Trifluoroacetylpteroic acid was conjugated to chemiluminescent N-sulfonylacridinium-9-carboxamide labels at the N(10) or 9-position carboxamide. Upon binding to folate binding protein the light output of the N(10) derivative (9) was quenched up to 62% upon triggering with basic peroxide, while the
H Oe et al.
Journal of nutritional science and vitaminology, 29(5), 523-531 (1983-10-01)
A sensitive radioassay method has been developed to quantitate the activity of the folate-hydrolyzing enzyme which catalyzes the hydrolysis of folic acid to pteroic acid and glutamic acid. The method is based on analyzing [2-14C]pteroic acid separated by a thin-layer
Application of halogeno-ketones to the synthesis of pteridines, including pteroic acid.
F E KING et al.
Nature, 164(4170), 574-574 (1949-10-01)
R L Stephens et al.
The Journal of pharmacology and experimental therapeutics, 239(3), 627-633 (1986-12-01)
Folic acid (FA) and 5-formyltetrahydrofolic acid (FTHF) have been shown previously to produce a marked stimulation of locomotor activity after bilateral injection into the rat nucleus accumbens. This study was designed to determine whether the hypermotility response produced by the
M J Akhtar et al.
Journal of pharmaceutical and biomedical analysis, 16(1), 95-99 (1998-02-03)
A high performance liquid chromatographic procedure was developed to determine folic acid and its photodegradation products, p-aminobenzoic acid, pterine-6-carboxylic acid, p-aminobenzoyl-L-glutamic acid, and pteroic acid in the presence of riboflavin. The method involves reversed phase, paired-ion chromatography on mu-BondaPak C18
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service