E40609
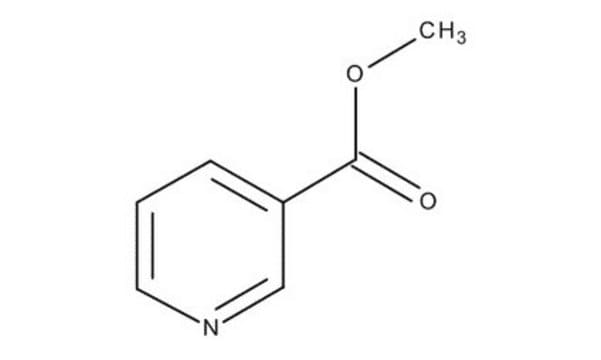
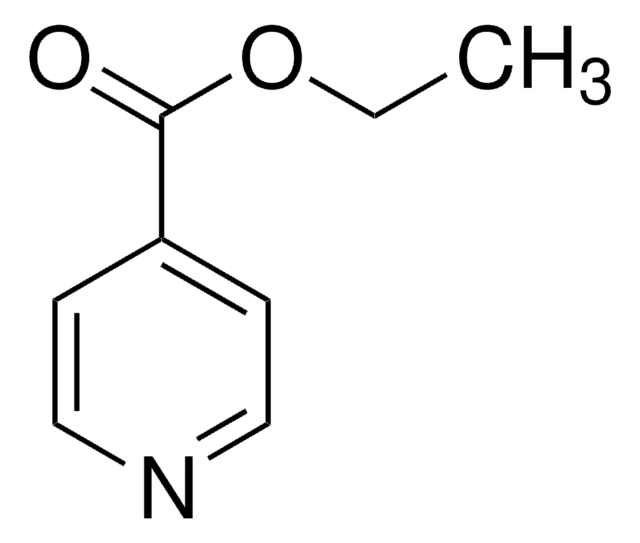
Ethyl nicotinate
99%
Synonym(s):
Nicotinic acid ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H9NO2
CAS Number:
Molecular Weight:
151.16
Beilstein:
122937
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.504 (lit.)
bp
223-224 °C (lit.)
mp
8-10 °C (lit.)
density
1.107 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)c1cccnc1
InChI
1S/C8H9NO2/c1-2-11-8(10)7-4-3-5-9-6-7/h3-6H,2H2,1H3
InChI key
XBLVHTDFJBKJLG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
199.4 °F - closed cup
Flash Point(C)
93 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K Sugibayashi et al.
Journal of controlled release : official journal of the Controlled Release Society, 62(1-2), 201-208 (1999-10-16)
An in vitro permeation study of ethyl nicotinate (EN) was carried out using excised hairless rat skin, and simultaneous skin transport and metabolism of the drug were kinetically followed. Fairly good steady-state fluxes of EN and its metabolite nicotinic acid
Tetsuya Hasegawa et al.
Biological & pharmaceutical bulletin, 31(1), 85-89 (2008-01-08)
The skin disposition and metabolism of topically applied ethyl nicotinate (EN) were evaluated in dual agar gel disc-inserted hairless rats, which have two agar gel discs subcutaneously inserted into the abdominal region as drug receptors, and a topical formulation containing
Ibrahim Uçar et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 72(1), 11-16 (2008-11-18)
Mononuclear copper(II) saccharinate (sac) complex containing ethylnicotinate (enc), [Cu(enc)(2)(sac)(2)(H(2)O)].1.4H(2)O has been synthesized and characterized by spectroscopic (IR, UV-vis, EPR), X-ray diffraction technique and electrochemical methods. It crystallizes in the tetragonal crystal systems with space group I4(1)cd and Z=8. The copper(II)
Zuleikha A M Nworgu et al.
Acta poloniae pharmaceutica, 64(2), 179-182 (2007-08-02)
3-Carbomethoxypyridine (CMP) was isolated and characterized from the leaves of Pyrenacantha staudtii Hutch and Dalz, family Icacinaceae, in our earlier study and was found to possess an inhibitory activity on the isolated rat uterus. In order to study the structure
Adel S Girgis et al.
European journal of medicinal chemistry, 43(9), 1818-1827 (2008-02-05)
2-(alicyclic-amino)-4,6-diaryl-3-pyridinecarboxylates 5a-d were prepared via aromatic nucleophilic substitution reaction of secondary amines (piperidine or morpholine) with 2-bromo-3-pyridinecarboxylate derivatives 3a,b. The latters were obtained through bromination of 3-aryl-4-benzoyl-2-cyanobutyrates 2a and 2b, which were obtained from the base promoted addition of ethyl
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service