All Photos(1)
About This Item
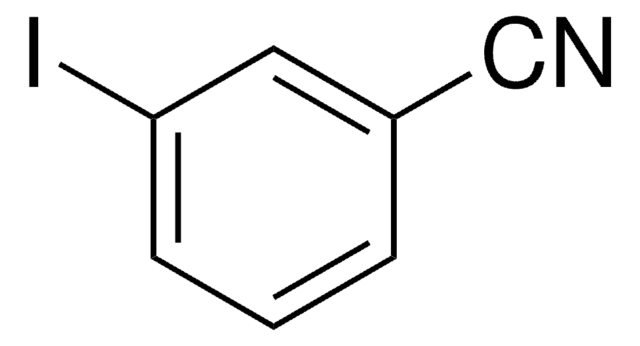
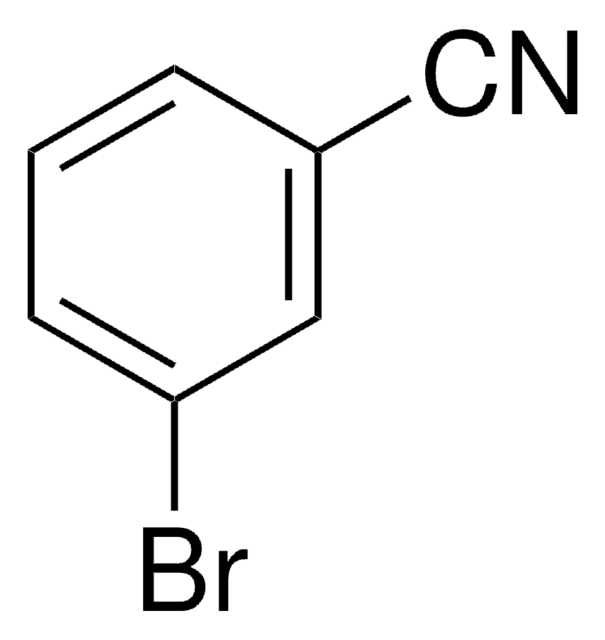
Linear Formula:
IC6H4CN
CAS Number:
Molecular Weight:
229.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
124-128 °C (lit.)
SMILES string
Ic1ccc(cc1)C#N
InChI
1S/C7H4IN/c8-7-3-1-6(5-9)2-4-7/h1-4H
InChI key
XOKDXPVXJWTSRM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
4-Iodobenzonitrile may be used in the synthesis of the following:
- ethyl-4′-cyano-4-nitro[1,1′-biphenyl]-2-carboxylate via a multi-step reaction process
- 2-(4-cyanophenyl)tellurophene obtained via Stille coupling with tellurophene
- 2,5-bis(4-cyanophenyl)tellurophene obtained via Stille coupling with 2,5-bis(trimethylstannyl)tellurophene
- ethyl-4′-cyano-6-{[(E)-phenylmethylidene]amino}[1,1′-biphenyl]-3-carboxylate via a multi-step reaction process
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
"Synthesis and stille cross-coupling reactions of 2-(tributylstannyl)-and 2, 5-bis (trimethylstannyl) tellurophene"
Sweat.PD and Stephens.EC
Synthesis, 2009(19), 3214-3218 (2009)
"Synthesis of Nitro-Substituted Polyfunctional Biphenyls by Negishi Cross-Coupling of o-Nitroarylzinc Reagents"
Sapountzis I, et al.
Advanced Synthesis & Catalysis, 346(07), 709-712 (2004)
"Preparation and reactions of functionalized arylmagnesium reagents"
Jensen EA, et al.
Synthesis, 2002(04), 0565-0569 (2002)
Nico Giordano et al.
Molecules (Basel, Switzerland), 24(10) (2019-05-30)
The crystal structure of 4-iodobenzonitrile, which is monoclinic (space group I2/a) under ambient conditions, contains chains of molecules linked through C≡N···I halogen-bonds. The chains interact through CH···I, CH···N and π-stacking contacts. The crystal structure remains in the same phase up
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service