All Photos(1)
About This Item
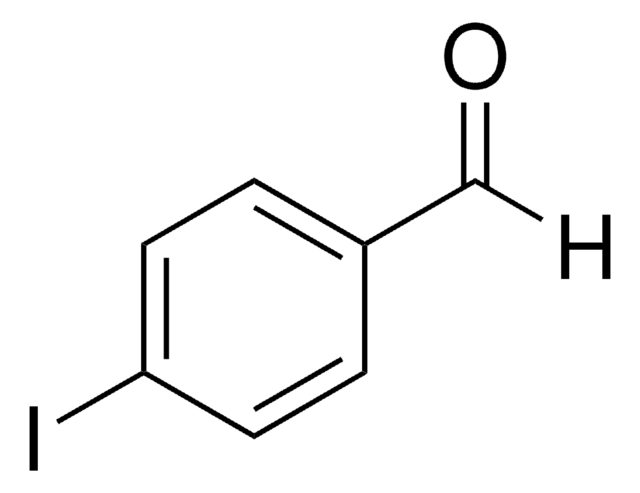
Linear Formula:
IC6H4CHO
CAS Number:
Molecular Weight:
232.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
36-39 °C (lit.)
storage temp.
2-8°C
SMILES string
Ic1ccccc1C=O
InChI
1S/C7H5IO/c8-7-4-2-1-3-6(7)5-9/h1-5H
InChI key
WWKKTHALZAYYAI-UHFFFAOYSA-N
General description
2-Iodobenzaldehyde (o-iodobenzaldehyde) is a 2-halobenzaldehyde derivative. Its crystals belong to the orthorhombic crystal system and P212121 space group.
Application
2-Iodobenzaldehyde may be used as a reactant in the synthesis of the following heterocycles:
- 2,3-diaryl-1-indenones
- indolo[1,2-a]quinazolines
- Baylis-Hillman (BH) adducts
- 5-phenylindazolo[3,2-b]quinazolin-7(5H)-one
- 4-(3-iodophenyl)-2,2:6,2-terpyridine
- fluoren-9-one
- 2-formyl-3′-methoxybiphenyl
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
2-Iodobenzaldehyde.
Betz R and Klufers P.
Acta Crystallographica Section E, Structure Reports Online, 63(12), o4879-o4879 (2007)
Ring-Closing Olefin Metathesis of 2, 2'-Divinylbiphenyls: A Novel and General Approach to Phenanthrenes.
Iuliano A, et al.
Organic Letters, 6(21), 3711-3714 (2004)
Copper (I)-Catalyzed Synthesis of 5-Arylindazolo [3, 2-b] quinazolin-7 (5 H)-one via Ullmann-Type Reaction
Chen DS, et al.
The Journal of Organic Chemistry, 78(11), 5700-5704 (2013)
A simple copper-catalyzed two-step one-pot synthesis of indolo [1, 2-a] quinazoline.
Li C, et al.
Beilstein Journal of Organic Chemistry, 10(1), 2441-2447 (2014)
Self-Assembly of Shape-Persistent Hexagonal Macrocycles with Trimeric Bis (terpyridine)-FeII Connectivity.
Li S, et al.
European Journal of Organic Chemistry, 2008(19), 3328-3334 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service