185493
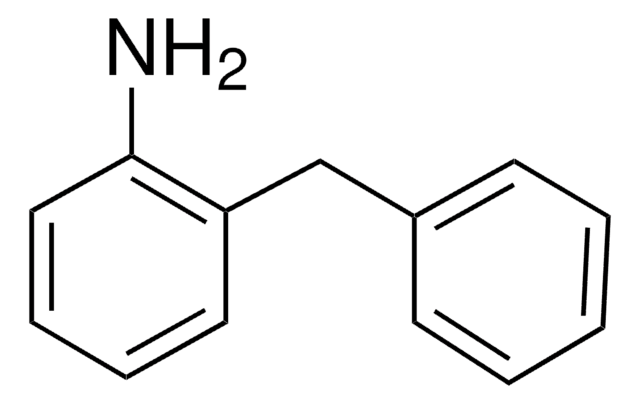
N-Benzylaniline
≥99%
Synonym(s):
N-Phenylbenzylamine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH2NHC6H5
CAS Number:
Molecular Weight:
183.25
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥99%
form
solid
bp
306-307 °C (lit.)
mp
35-38 °C (lit.)
solubility
alcohol: soluble
chloroform: soluble
diethyl ether: soluble
water: insoluble
density
1.061 g/mL at 25 °C (lit.)
functional group
amine
phenyl
SMILES string
C(Nc1ccccc1)c2ccccc2
InChI
1S/C13H13N/c1-3-7-12(8-4-1)11-14-13-9-5-2-6-10-13/h1-10,14H,11H2
InChI key
GTWJETSWSUWSEJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
The electropolymerisation of N-benzylaniline at transparent Indium Tin Oxide glass electrodes has been investigated by UV-visible spectroelectrochemistry. N-Benzylaniline on electrochemical oxidation in aqueous sulfuric acid solution produces an adherent conducting polymer film at the platinum electrode.
Application
N-Benzylaniline was used in the separation of tervalent gallium, indium and thallium by solvent extraction method.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M M Khosla et al.
Talanta, 21(6), 411-415 (1974-06-01)
A simple and rapid method is proposed for the separation of tervalent gallium, indium and thallium by solvent extraction with N-benzylaniline in chloroform from different concentrations of hydrochloric acid. Thallium and gallium are extracted from 1M and 7.0-7.5M hydrochloric acid
A UV-visible spectroelectrochemical study of the electropolymerisation of N-benzylaniline.
Malinauskas A and Holze R.
Journal of Solid State Electrochemistry, 3(7-8), 429-436 (1999)
Identification of a new metabolite after incubation of N-benzylaniline with rabbit liver microsomes.
H M Ali et al.
Journal of chromatography, 202(2), 287-293 (1980-12-19)
Yung-Hung Chang et al.
Dalton transactions (Cambridge, England : 2003), (5)(5), 861-867 (2009-01-22)
Both saturated and unsaturated N-benzyl substituted heterocyclic carbene (NHC) iridum(i) complexes were synthesized. The unsaturated carbene complex [(un-NHC-Bn)Ir(CO)(2)Cl] in the cis form was prepared via the carbene transfer from the corresponding silver complex to [Ir(COD)(2)Cl](2) followed by ligand substitution with
Masaharu Uno et al.
Organic & biomolecular chemistry, 6(6), 979-981 (2008-03-11)
N-Benzylanilines were designed and synthesized as vascular endothelial growth factor (VEGF)-2 inhibitors using de novo drug design systems based on the X-ray structure of VEGFR-2 kinase domain. Among compounds synthesized, compound showed the most potent inhibitory activity toward VEGFR-2 (KDR)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service