All Photos(4)
About This Item
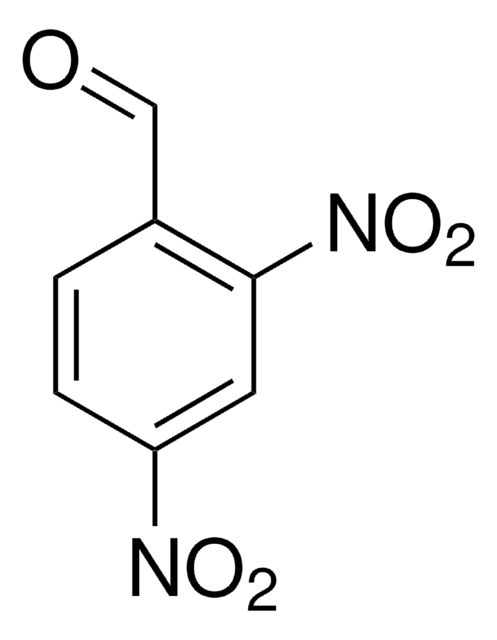
Linear Formula:
(O2N)2C6H3CH2OH
CAS Number:
Molecular Weight:
198.13
Beilstein:
2054388
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
88-91 °C (lit.)
functional group
hydroxyl
nitro
SMILES string
OCc1cc(cc(c1)[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C7H6N2O5/c10-4-5-1-6(8(11)12)3-7(2-5)9(13)14/h1-3,10H,4H2
InChI key
GPHYIQCSMDYRGJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3,5-Dinitrobenzyl alcohol on reaction with p-toluenesulphonyl chloride yields 3,5-dinitrobenzyl p-toluenesulphonate.
Application
3,5-Dinitrobenzyl alcohol was used in the synthesis of 3,5-bis((bezoxycarbonyl)imino)benzyl alcohol.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis and characterization of hyperbranched polyurethanes prepared from blocked isocyanate monomers by step-growth polymerization.
Spindler R and Frechet JMJ.
Macromolecules, 26(18), 4809-4813 (1993)
Ludmila Eisner et al.
Sensors (Basel, Switzerland), 19(18) (2019-09-13)
A sensor for trinitrotoluene (TNT) detection was developed by using a combination of optical micro-ring technology and a receptor coating based on molecularly imprinted sol-gel layers. Two techniques for deposition of receptor layers were compared: Airbrush technology and electrospray ionization.
K Funazo et al.
Journal of chromatography, 481, 211-219 (1989-11-03)
New UV-labelling agents have been synthesized, which are designed to convert monocarboxylic acids into their highly UV-absorbing derivatives for enhancement of the sensitivities of UV detection in high-performance liquid chromatography. The reagents are p-nitrobenzyl, 3,5-dinitrobenzyl and 2-(phthalimino)ethyl p-toluenesulphonates. Each has
Evon Powell et al.
International journal of nanomedicine, 2(3), 449-459 (2007-11-21)
The interaction of the important but often overdosed local anesthetic bupivacaine, its structural analogs 2,6-dimethylaniline, and N-methyl-2,6-dimethylacetanilide, and cocaine, with several electron deficient aromatic moieties were studied primarily by proton NMR and UV-visible spectroscopy. In solution, the anesthetic, its analogs
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)