All Photos(1)
About This Item
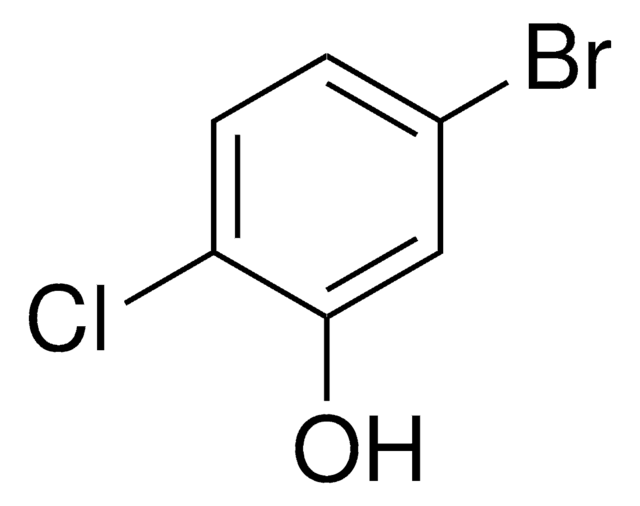
Linear Formula:
BrC6H3(Cl)OH
CAS Number:
Molecular Weight:
207.45
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
fibers
bp
232-235 °C (lit.)
mp
47-49 °C (lit.)
functional group
bromo
chloro
SMILES string
Oc1ccc(Br)cc1Cl
InChI
1S/C6H4BrClO/c7-4-1-2-6(9)5(8)3-4/h1-3,9H
InChI key
VIBJPUXLAKVICD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4-Bromo-2-chlorophenol is biologically inactive metabolite of profenofos, an organophosphorus pesticide. It undergoes enzyme-catalyzed copolymerization with phenols catalyzed by extracellular laccase of the fungus Rhizoctonia praticola.
Application
4-Bromo-2-chlorophenol was used as reagent during the synthesis of 7-arylbenzo
[b][1,4]oxazin derivatives.
[b][1,4]oxazin derivatives.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Solid-phase Synthesis of 7-Aryl-benzo [b][1, 4] oxazin-3 (4H)-one Derivatives on a BOMBA Resin Utilizing the Smiles Rearrangement.
Lee JM, et al.
Bull. Korean Chem. Soc., 30(6), 1325-1330 (2009)
J J Sanchez Saez et al.
Food additives and contaminants, 8(5), 627-631 (1991-09-01)
An off-odour, described by the growers as similar to profenofos, occurred in melons in which this pesticide had been used in crop treatment. However, profenofos, O-(4-bromo-2-chlorophenyl) O-ethyl S-propyl phosphorothioate, could not be detected in the melons using GC/MS although a
Mingbo Ma et al.
Journal of environmental science and health. Part. B, Pesticides, food contaminants, and agricultural wastes, 54(1), 70-75 (2019-01-12)
Pesticides carried by cotton fiber are potential risk for production workers and consumers. Dissipation behaviour of a commonly used cotton pesticide profenofos in cotton fiber during growing period and scouring treatment was investigated. The results showed that profenofos in the
Copolymerization of halogenated phenols and syringic acid.
Bollag J-M and Liu S-Y.
Pesticide Biochemistry and Physiology, 23(2), 261-272 (1985)
Oswald A Dadson et al.
Toxicology, 306, 35-39 (2013-02-19)
Profenofos is a direct acting phosphorothioate organophosphorus (OP) pesticide capable of inhibiting β-esterases such as acetylcholinesterase, butyrylcholinesterase, and carboxylesterase. Profenofos is known to be detoxified to the biologically inactive metabolite, 4-bromo-2-chlorophenol (BCP); however, limited data are available regarding the use
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service