105449
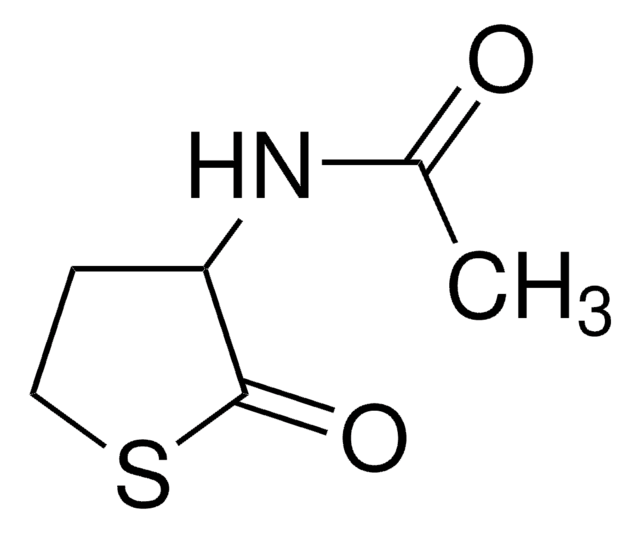
γ-Thiobutyrolactone
98%
Synonym(s):
4-Butyrothiolactone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6OS
CAS Number:
Molecular Weight:
102.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
refractive index
n20/D 1.523 (lit.)
bp
39-40 °C/1 mmHg (lit.)
solubility
THF: soluble
density
1.18 g/mL at 25 °C (lit.)
functional group
thioester
SMILES string
O=C1CCCS1
InChI
1S/C4H6OS/c5-4-2-1-3-6-4/h1-3H2
InChI key
KMSNYNIWEORQDJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
γ-Thiobutyrolactone undergoes copolymerization with glycidyl phenyl ether to form poly(ester-alt-sulfide).
Application
γ-Thiobutyrolactone was used to terminate the ring opening polymerization of ω-pentadecalactone to synthesize difunctional polyesters. γ-Thiobutyrolactone was used to study the mechanism of metabolism of sulphur containing heterocyclic compounds by lignin-degrading basidiomycete Coriolus versicolor.
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
186.8 °F - closed cup
Flash Point(C)
86 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
K D Holland et al.
Brain research, 615(1), 170-174 (1993-06-25)
Effects of alkyl-substituted gamma-butyrolactones and gamma-thiobutyrolactones on [35S]t-butylbicyclophosphorothionate (35S-TBPS) dissociation from the picrotoxinin receptor were studied. Unlike picrotoxinin, these lactones accelerated the dissociation rate of 35S-TBPS. Thus, previous reports that these lactones change the Kd but not the Bmax of
Nishikubo et al.
Macromolecules, 31(15), 4746-4752 (1998-07-29)
Poly(ester-alt-sulfide) (polymer 1) was synthesized by the alternating copolymerization of glycidyl phenyl ether (GPE) with gamma-thiobutyrolactone (TBL) catalyzed by either quaternary onium salts or crown ether complexes. The copolymerization proceeded to produce polymer 1 with good yields in neat or
One-pot difunctionalization of poly (ω-pentadecalactone) with thiol-thiol or thiol-acrylate groups, catalyzed by Candida antarctica lipase B.
Takwa M, et al.
Macromolecular Rapid Communications, 27(22), 1932-1936 (2006)
Md Abdul Kafi et al.
Regenerative biomaterials, 7(2), 141-151 (2020-04-17)
Scaffold engineering has attracted significant attention for three-dimensional (3D) growth, proliferation and differentiation of stem cells in vitro. Currently available scaffolds suffer from issues such as poor ability for cell adhesion, migration and proliferation. This paper addresses these issues with
Jonathan Garel et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(15), 4144-4152 (2006-02-03)
Homocysteine thiolactone (tHcy) is deemed a risk factor for cardiovascular diseases and strokes, presumably because it acylates the side chain of protein lysine residues ("N-homocysteinylation"), thereby causing protein damage and autoimmune responses. We analysed the kinetics of hydrolysis and aminolysis
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service