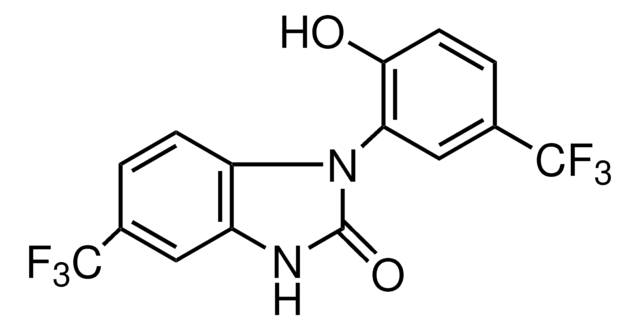
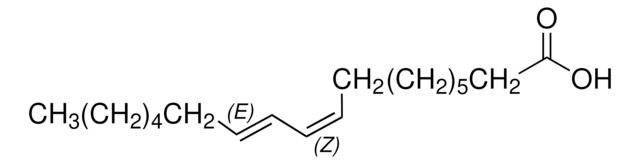
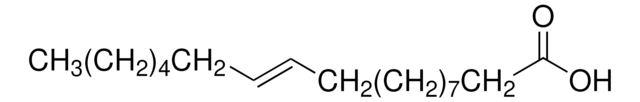
O5632
Linoleic acid, conjugated methyl ester
Synonym(s):
CLA methyl ester, Octadecadienoic acid, conjugated, methyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
biological source
plant (safflower)
Assay
≥99% (GC)
form
liquid
technique(s)
GC/MS: suitable
HPLC: suitable
impurities
≤1% Linoleic acid methyl ester
functional group
carboxylic acid
ester
lipid type
omega FAs
shipped in
ambient
storage temp.
−20°C
Related Categories
General description
Conjugated linoleic acid (CLA) describes the positional and geometric isomers of C18:2 fatty acids (FA) with a conjugated double bond. cis-9, trans-11 CLA are considered as the major isomers of CLA, based on in vitro experiments and animal studies in milk. The major source of CLA in human diet is the ruminant fat.
Application
Linoleic acid, conjugated methyl ester (CLA) has been used as a standard for the determination of isomers of CLA in milk samples by high performance liquid chromatography (HPLC), gas chromatography-mass spectrometry. It has also been used in the production of sn-1,3 diacyl glycerol.
Biochem/physiol Actions
Conjugated linoleic acid (CLA) isomers as supplements inhibit the growth of numerous human cancer cell lines. It also possesses immunostimulant properties, prevents diabetes and reduces atherosclerosis.
Other Notes
A mixture of cis- and trans-9,11- and -10,12-octadecadienoic acid methyl esters. Isomer ratio may vary from lot to lot.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P G Toral et al.
Journal of dairy science, 101(7), 6109-6121 (2018-05-01)
A great deal of uncertainty still exists about intermediate metabolites and pathways explaining the biohydrogenation (BH) of 20- and 22-carbon polyunsaturated fatty acids (PUFA). Therefore, this study was conducted to provide further insight into the ruminal metabolism of 20:5 n-3
A D Martin et al.
Journal of dairy science, 98(8), 5374-5384 (2015-05-26)
To investigate the feasibility of milk fatty acids as predictors of onset of luteal activity (OLA), 87 lactations taken from 73 healthy Norwegian Red cattle were surveyed over 2 winter housing seasons. The feasibility of using frozen milk samples for
M Coppa et al.
Journal of dairy science, 102(11), 10483-10499 (2019-09-10)
The aims of this work were to determine the effect of upland origin on milk composition when comparing similar lowland and upland production system and to highlight the factors responsible for the added value of upland milk from commercial farms.
Examination of the persistency of milk fatty acid composition responses to plant oils in cows given different basal diets, with particular emphasis on trans-C 18: 1 fatty acids and isomers of conjugated linoleic acid
Roy A, et al.
Animal Science Journal = Nihon Chikusan Gakkaiho, 82(4), 479-492 (2006)
Ali Karabacak et al.
TheScientificWorldJournal, 2014, 821904-821904 (2014-02-14)
This study was conducted to compare fatty acid composition and content of conjugated linoleic acid (CLA) in different regions of sheep carcasses. Lambs of the Dağlıç breed were used for this purpose. Subsequent to a 68-day period of intensive fattening
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service