D102504
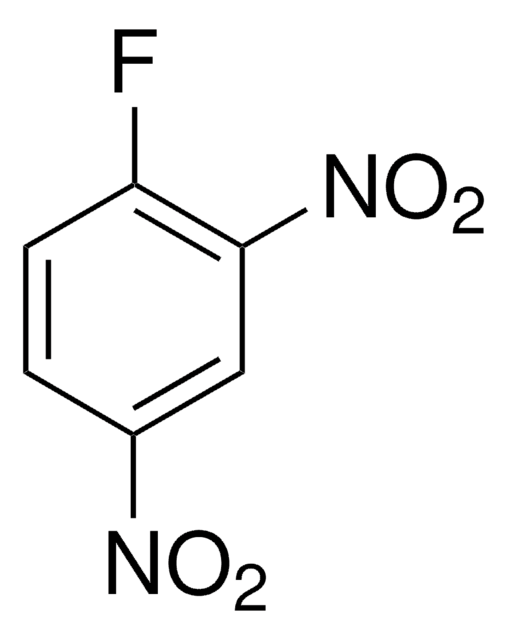
1,5-Difluoro-2,4-dinitrobenzene
97%
Synonym(s):
DFDNB
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
F2C6H2(NO2)2
CAS Number:
Molecular Weight:
204.09
Beilstein:
1883116
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
72-74 °C (lit.)
SMILES string
[O-][N+](=O)c1cc(c(F)cc1F)[N+]([O-])=O
InChI
1S/C6H2F2N2O4/c7-3-1-4(8)6(10(13)14)2-5(3)9(11)12/h1-2H
InChI key
VILFTWLXLYIEMV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - STOT RE 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yanshun Liu et al.
Protein science : a publication of the Protein Society, 11(2), 371-380 (2002-01-16)
When concentrated in mildly acidic solutions, bovine pancreatic ribonuclease (RNase A) forms long-lived oligomers including two types of dimer, two types of trimer, and higher oligomers. In previous crystallographic work, we found that the major dimeric component forms by a
Alexander Shivanyuk et al.
Organic letters, 4(9), 1555-1558 (2002-04-27)
[reaction: see text]. o-alkylation of C2V-symmetrical resorcinarene tetraesters 2 with 2 equiv of 1,3-difluoro-4,6-dinitrobenzene readily affords conformationally rigid octanitro resorcinarene 3, which is a potential scaffold for the design of supramolecular structures.
Zhanguo Wang et al.
Journal of combinatorial chemistry, 9(4), 652-660 (2007-05-17)
This paper discusses the synthesis of privileged structures 4H-benzo[1,4]thiazin-3-one and 1,1-dioxo-1,4-dihydro-2H-1lambda6-benzo[1,4]thiazin-3-one derivatives in a parallel solution-phase manner using 1,5-difluoro-2,4-dinitrobenzene. Each scaffold possesses four diversity points. A cheap and efficient oxidant, urea-hydrogen peroxide (UHP), was applied for the introduction of the
S Simonsson et al.
The Journal of biological chemistry, 273(38), 24633-24639 (1998-09-12)
The UL9 gene of herpes simplex virus type 1 (HSV-1) encodes an origin binding protein (OBP). It is an ATP-dependent DNA helicase and a sequence-specific DNA-binding protein. The latter function is carried out by the C-terminal domain of OBP (DeltaOBP).
Yunyun Yuan et al.
Journal of combinatorial chemistry, 9(1), 158-170 (2007-01-09)
This paper discusses the synthesis of benzo[1,4]oxazin-3-one-based compounds from 1,5-difluoro-2,4-dinitrobenzene (1), including benzo[1,4]oxazin-3-ones (5-11) and five novel benzo[1,4]oxazin-3-one-based tricycles: 6-hydroxy-4H-1-oxa-4,5,8-triazaanthracen-3-one (14), 3,8-dihydro-5-oxa-1,3,8-triazacyclopenta-[b]-naphthalene-7-one (15, 17, 21), 3,8-dihydro-5-oxa-1,2,3,8-tetraazacylopenta[b]-naphthalene-7-one (16, 20), 3,8-dihydro-1H-5-oxa-1,3,8-triazacyclopenta[b]-naphthalene-2,7-dione (18, 22), and 5,8-dihydro-4H-1-oxa-4,5,8-triazaanthracene-3,6,7-trione (19). Finally, a chemical library based on
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)






