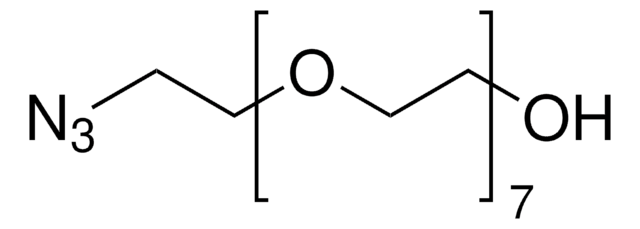
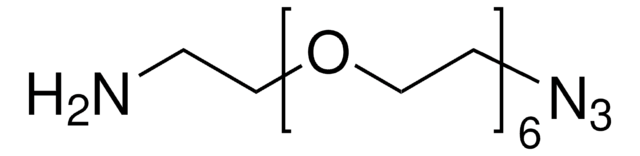
776130
3-Azido-1-propanol
≥96%
Synonym(s):
1-Azidopropan-3-ol
About This Item
Recommended Products
Assay
≥96%
form
liquid
reaction suitability
reaction type: click chemistry
refractive index
n20/D 1.461
density
1.095 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
OCCCN=[N+]=[N-]
InChI
1S/C3H7N3O/c4-6-5-2-1-3-7/h7H,1-3H2
InChI key
WHVSIWLMCCGHFW-UHFFFAOYSA-N
General description
Application
- Precursor in the synthesis of heterocyclic compounds like dihydrooxazines
- Reagent in the synthesis of heterofunctional polyesters by 1,3-dipolar cycloaddition
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Drug discovery process by utilizing chemistry reaction of Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of terminal alkynes with organoazides to yield 1,4-disubstituted 1,2,3-triazoles.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![2-[2-(2-Azidoethoxy)ethoxy]ethanol solution ~0.5 M in tert-butyl methyl ether](/deepweb/assets/sigmaaldrich/product/structures/374/007/eea7ca74-41e4-4aac-af71-c93c37ec0a5a/640/eea7ca74-41e4-4aac-af71-c93c37ec0a5a.png)