All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H11N
CAS Number:
Molecular Weight:
85.15
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
refractive index
n20/D 1.4353
Quality Level
density
0.842 g/mL at 25 °C (lit.)
storage temp.
2-8°C
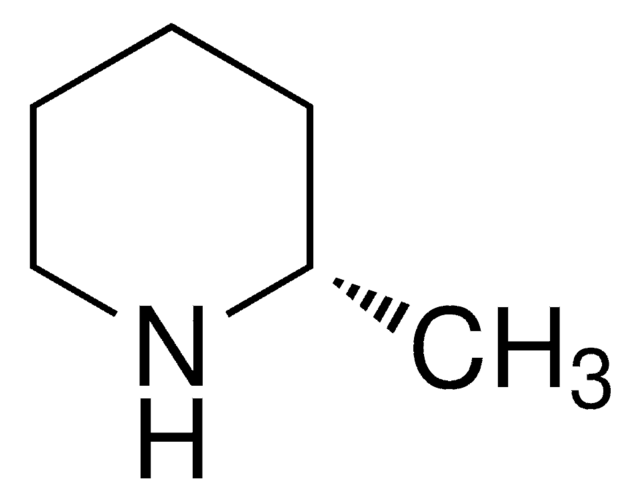
SMILES string
C[C@@H]1CCCN1
InChI
1S/C5H11N/c1-5-3-2-4-6-5/h5-6H,2-4H2,1H3/t5-/m1/s1
InChI key
RGHPCLZJAFCTIK-RXMQYKEDSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
(R)-(−)-2-Methylpyrrolidine, an optically active amine that can be used as a key building block to synthesize:
- 4,5-fused pyridazinone derivatives are applicable as potent histamine H3 receptor antagonists.
- Naphthalenoid histamine H3 receptor antagonist.
- Pyrrolo[2,3-d]pyrimidine derivatives as potent inhibitors of leucine-rich repeat kinase 2.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
45.0 °F - closed cup
Flash Point(C)
7.22 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
In vitro SAR of pyrrolidine-containing histamine H 3 receptor antagonists: Trends across multiple chemical series
Nersesian, Diana L., et al.
Bioorganic & Medicinal Chemistry Letters, 18.1, 355-359 (2008)
Optical Activation of Racemic a-Substituted Carbonyl Compounds Using Optically Active Amines.
Matsushita H, et al.
Bulletin of the Chemical Society of Japan, 49(7), 1928-1930 (1976)
An expedient and multikilogram synthesis of a naphthalenoid H3 antagonist.
Pu YM, et al.
Organic Process Research & Development, 11(6), 1004-1009 (2007)
Douglas S Williamson et al.
Journal of medicinal chemistry, 64(14), 10312-10332 (2021-06-30)
Inhibitors of leucine-rich repeat kinase 2 (LRRK2) and mutants, such as G2019S, have potential utility in Parkinson's disease treatment. Fragment hit-derived pyrrolo[2,3-d]pyrimidines underwent optimization using X-ray structures of LRRK2 kinase domain surrogates, based on checkpoint kinase 1 (CHK1) and a
Ming Tao et al.
Bioorganic & medicinal chemistry letters, 21(20), 6126-6130 (2011-09-13)
Three series of novel 4,5-fused pyridazinones were synthesized as histamine H(3) receptor antagonists. The 2,5,6,7-tetrahydrocyclopenta[d]pyridazin-1-one 5q and 5,6,7,8-tetrahydro-2H-phthalazin-1-one 5u displayed high affinity at both rat and human H(3) receptors, and showed potent antagonist and full inverse agonist activity in functional
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service