V1377
Vinblastine sulfate salt
≥97% (HPLC), powder, plant alkaloid
Synonyme(s) :
VLB, Vincaleukoblastine sulfate salt
About This Item
Produits recommandés
product name
Vinblastine sulfate salt, ≥97% (HPLC)
Niveau de qualité
Pureté
≥97% (HPLC)
Forme
(powder or amorphous or crystalline powder)
Couleur
white to light yellow
Pf
267 °C (dec.) (lit.)
Absorption
14 at 270 nm in 0.1 M phosphate buffer at 1 mM
16.2 at 259 nm in ethanol at 1 mM
53.7 at 214 nm in ethanol at 1 mM
Spectre d'activité de l'antibiotique
neoplastics
Mode d’action
DNA synthesis | interferes
Auteur
Eli Lilly
Température de stockage
2-8°C
Chaîne SMILES
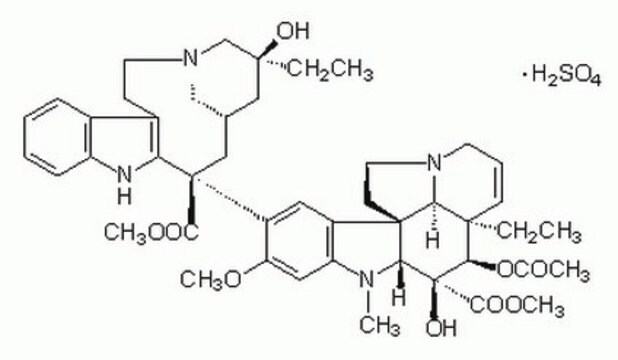
OS(O)(=O)=O.[H][C@@]12CN(CCc3c([nH]c4ccccc34)[C@@](C1)(C(=O)OC)c5cc6c(cc5OC)N(C)[C@@]7([H])[C@](O)([C@H](OC(C)=O)[C@]8(CC)C=CCN9CC[C@]67[C@]89[H])C(=O)OC)C[C@](O)(CC)C2
InChI
1S/C46H58N4O9.H2O4S/c1-8-42(54)23-28-24-45(40(52)57-6,36-30(15-19-49(25-28)26-42)29-13-10-11-14-33(29)47-36)32-21-31-34(22-35(32)56-5)48(4)38-44(31)17-20-50-18-12-16-43(9-2,37(44)50)39(59-27(3)51)46(38,55)41(53)58-7;1-5(2,3)4/h10-14,16,21-22,28,37-39,47,54-55H,8-9,15,17-20,23-26H2,1-7H3;(H2,1,2,3,4)/t28-,37-,38+,39+,42-,43+,44+,45-,46-;/m0./s1
Clé InChI
KDQAABAKXDWYSZ-PNYVAJAMSA-N
Informations sur le gène
human ... TBCC(6903) , TUBA1A(7846) , TUBA1B(10376) , TUBA1C(84790) , TUBA3C(7278) , TUBA3E(112714) , TUBA4A(7277) , TUBB(203068) , TUBB1(81027) , TUBB2A(7280) , TUBB2B(347733) , TUBB3(10381) , TUBB4A(10382) , TUBB4B(10383) , TUBB6(84617) , TUBB8(347688)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Application
- as a microtubule depolymerizing drug for the synchronization of human cell lines in G2/M phase
- as a multidrug resistance screening substrate in human colon cancer cell line (HCT116) cell line
- as an antimicrotubule agent in sub perineural glia of Drosophila brain
Actions biochimiques/physiologiques
Caractéristiques et avantages
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Muta. 2 - Repr. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique