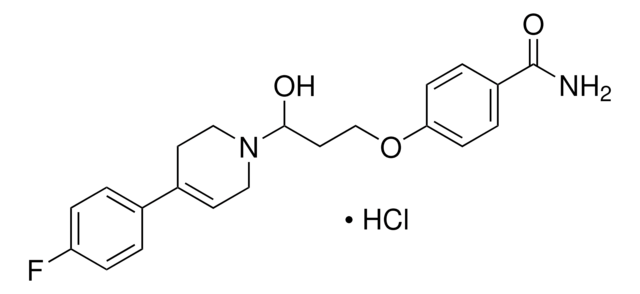
I119
Indatraline hydrochloride
solid
Synonyme(s) :
(±)-trans-3-(3,4-Dichlorophenyl)-N-methyl-1-indanamine hydrochloride, Lu 19-005
About This Item
Produits recommandés
Forme
solid
Niveau de qualité
Couleur
white
Solubilité
H2O: 2 mg/mL
Température de stockage
2-8°C
Chaîne SMILES
Cl.CN[C@@H]1C[C@@H](c2ccc(Cl)c(Cl)c2)c3ccccc13
InChI
1S/C16H15Cl2N.ClH/c1-19-16-9-13(11-4-2-3-5-12(11)16)10-6-7-14(17)15(18)8-10;/h2-8,13,16,19H,9H2,1H3;1H/t13-,16+;/m0./s1
Clé InChI
QICQDZXGZOVTEF-MELYUZJYSA-N
Informations sur le gène
human ... DRD1(1812) , DRD2(1813) , DRD3(1814) , DRD4(1815) , DRD5(1816) , HTR1A(3350) , HTR1B(3351) , HTR1D(3352) , HTR1E(3354) , HTR1F(3355) , HTR2A(3356) , HTR2B(3357) , HTR2C(3358) , HTR3A(3359) , HTR3B(9177) , HTR3C(170572) , HTR3D(200909) , HTR3E(285242) , HTR4(3360) , HTR5A(3361) , HTR5B(645694) , HTR6(3362) , HTR7(3363)
Application
- as a competitive inhibitor of 3H-dopamine ([3H]DA) to study its effects on trans-activator of transcription (Tat) protein on cocaine-induced inhibition of uptake of [3H]DA
- as a dopamine transport blocker to study its effects on trace amine-associated receptor 1 (TAAR1)-transfected mice cells
- as a nonselective monoamine transport inhibitor to study its anti-angiogenic activities in glioblastoma multiforme (GBM)
Actions biochimiques/physiologiques
Caractéristiques et avantages
Informations légales
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Acute 1 - Aquatic Chronic 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Gloves
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique