G6142
Glycerokinase from Cellulomonas sp.
lyophilized powder, 25-75 units/mg protein
Synonyme(s) :
glpK, ATP:glycerol 3-phosphotransferase, Glycerol Kinase
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Forme
lyophilized powder
Niveau de qualité
Activité spécifique
25-75 units/mg protein
Poids mol.
~128 kDa (by gel filtration)
Composition
Protein, ≥60% biuret
Température de stockage
−20°C
Description générale
Research area: Cell Signaling
Glycerol kinase (GK) is part of the FGGY carbohydrate kinase family.
Glycerol kinase (GK) is part of the FGGY carbohydrate kinase family.
Application
Glycerokinase from Cellulomonas sp. has been used:
- for determining the kinetic characteristics of human and trypanosomatid phosphofructokinases using an enzyme-linked kinetic assay.
- to study the effect of sugar in fluorescence emission.
- in 2-Arachidonoylglycerol-based fluorescence assay for DH-463, a fluorescent activity-based probe for monoacylglycerol lipase.
Actions biochimiques/physiologiques
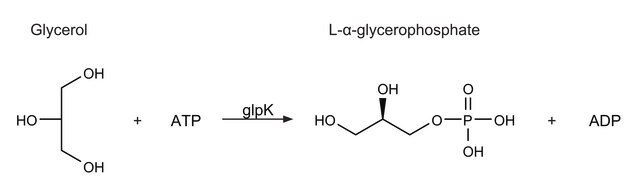
Glycerol kinase catalyzes the MgATP-dependent phosphorylation of glycerol to produce sn-glycerol-3-phosphate and is the rate limiting enzyme in the utilization of glycerol. It is also subject to feedback regulation by fructose-1,6-bisphosphate.Mutations in this gene are associated with Glycerol Kinase Deficiency (GKD), a condition characterized primarily by hypertriglyceridemia and hypoglycemia. This enzyme is useful for enzymatic determination of glycerol and triglyceride when coupled with glycerol-3-phosphate dehydrogenase (=G-3-P DH, G3D-301), glycerol-3-phosphate oxidase (=G-3-P oxidase, G3O-301, G3O-311, G3O-321) or pyruvate kinase (PYK-301) and lactate dehydrogenase (LCD-209, LCD-211), lipoprotein lipase (LPL-311, LPL-314) in clinical analysis
Propriétés physiques
Isoelectric point : 4.2
Michaelis constants : 4.4 x 10-5M (Glycerol), 4.3 x 10-4M (ATP)
Inhibitors : p-Chloromercuribenzoate, heavy metal ions (Pb++, Fe++, Hg++, Ag+)
Optimum pH : 9.8 (G-3-PDH system), 7.8 (G-3-P oxidase system) Optimum temperature : 500C
pH Stability : pH 5.5 x 10.0 (25oC, 20hr)
Thermal stability : below 40oC (pH 7.5, 15min)
Substrate specificity : This enzyme catalyzes the stereospecific transfer of the terminal
phosphoryl moiety of ATP to one of the primary hydroxyl group of
glycerol, forming sn-glycerol-3-P. The enzyme has the highest
specificity for glycerol, and also phosphorylates dihydroxyacetone
and glyceraldehyde (Table 1,2). Mg++ is essentially required for the
reaction.
Michaelis constants : 4.4 x 10-5M (Glycerol), 4.3 x 10-4M (ATP)
Inhibitors : p-Chloromercuribenzoate, heavy metal ions (Pb++, Fe++, Hg++, Ag+)
Optimum pH : 9.8 (G-3-PDH system), 7.8 (G-3-P oxidase system) Optimum temperature : 500C
pH Stability : pH 5.5 x 10.0 (25oC, 20hr)
Thermal stability : below 40oC (pH 7.5, 15min)
Substrate specificity : This enzyme catalyzes the stereospecific transfer of the terminal
phosphoryl moiety of ATP to one of the primary hydroxyl group of
glycerol, forming sn-glycerol-3-P. The enzyme has the highest
specificity for glycerol, and also phosphorylates dihydroxyacetone
and glyceraldehyde (Table 1,2). Mg++ is essentially required for the
reaction.
Définition de l'unité
One unit will convert 1.0 μmole of glycerol and ATP to L-α-glycerophosphate and ADP per min at pH 9.8 at 25 °C in a coupled system with PK/LDH.
Forme physique
Lyophilized powder containing phosphate buffer salts and sodium gluconate
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Resp. Sens. 1
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Peter M Fernandes et al.
The Biochemical journal, 476(2), 179-191 (2018-11-09)
Eukaryotic ATP-dependent phosphofructokinases (PFKs) are often considered unidirectional enzymes catalysing the transfer of a phospho moiety from ATP to fructose 6-phosphate to produce ADP and fructose 1,6-bisphosphate. The reverse reaction is not generally considered to occur under normal conditions and
Fei Ying et al.
Scientific reports, 14(1), 3922-3922 (2024-02-17)
The influence of lipid metabolism on tumorigenesis and progression has garnered significant attention. However, the role of Glycerol Kinase (GK), a key enzyme in glycerol metabolism, in Esophageal Carcinoma (ESCA) remains unclear. To further elucidate the relationship between GK and
N Zwaig et al.
Journal of bacteriology, 102(3), 753-759 (1970-06-01)
The activity of glycerol kinase is rate-limiting in the metabolism of glycerol by cells of Escherichia coli. A mutant strain producing a glycerol kinase resistant to inhibition by fructose-1,6-diphosphate grows faster than its wild-type parent on glycerol as the sole
Zhongya Qin et al.
Biomedical optics express, 9(7), 3373-3390 (2018-07-10)
The femtosecond laser ablation in biological tissue produces highly fluorescent compounds that are of great significance for intrinsically labelling ablated tissue in vivo and achieving imaging-guided laser microsurgery. In this study, we analyzed the molecular structures of femtosecond laser-ablated tissues
Purification and properties of glycerol kinase from Escherichia coli.
S I Hayashi et al.
The Journal of biological chemistry, 242(5), 1030-1035 (1967-03-10)
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique