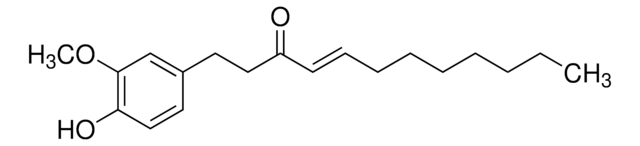
G1046
[6]-Gingerol
≥98% (HPLC)
Synonyme(s) :
3-Decanone, 5-hydroxy-1-(4-hydroxy-3-methoxyphenyl)-, (5S)-, 5-Hydroxy-1-(4-hydroxy-3-methoxyphenyl)-3-decanone, 6-Gingerol
About This Item
Produits recommandés
Niveau de qualité
Essai
≥98% (HPLC)
Conditions de stockage
protect from light
under inert gas
Solubilité
methanol: 1 mg/mL, clear, colorless
Application(s)
metabolomics
vitamins, nutraceuticals, and natural products
Conditions d'expédition
dry ice
Température de stockage
−20°C
Chaîne SMILES
CCCCC[C@H](O)CC(=O)CCc1ccc(O)c(OC)c1
InChI
1S/C17H26O4/c1-3-4-5-6-14(18)12-15(19)9-7-13-8-10-16(20)17(11-13)21-2/h8,10-11,14,18,20H,3-7,9,12H2,1-2H3/t14-/m0/s1
Clé InChI
NLDDIKRKFXEWBK-AWEZNQCLSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- to study its effects on transient receptor potential (TRP) channels
- to study its effects on experimental models of non-alcoholic steatohepatitis
- to determine its effects on microsomal prostaglandine E2 synthase 1 (mPGES-1), glycogen synthase kinase 3β (GSK-3β) and β-catenin pathway in A549 cell line
- to analyse the effects of 6-Shogaol (6-SG) on diabetic nephropathy (DN) in db/db mice
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique![[6]-Gingerol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/259/444/6877889c-1cc0-47f5-b807-f847deadec1d/640/6877889c-1cc0-47f5-b807-f847deadec1d.png)
![[6]-Gingerol, Zingiber officinale An antitumor, apoptosis-inducing compound of the ginger family that blocks EGF-induced cell transformation by inhibiting the activation of Activator Protein-1 (AP-1).](/deepweb/assets/sigmaaldrich/product/images/140/919/0846df46-0b67-4c28-b99d-87e177be65b2/640/0846df46-0b67-4c28-b99d-87e177be65b2.jpg)
![[8]-Gingerol analytical standard](/deepweb/assets/sigmaaldrich/product/structures/408/530/af2f2837-3419-4e07-a72b-24e95af0d7ce/640/af2f2837-3419-4e07-a72b-24e95af0d7ce.png)
![[10]-Gingerol ≥98% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/224/210/b4f3e699-03b9-4112-89c1-a63f196344d0/640/b4f3e699-03b9-4112-89c1-a63f196344d0.png)