D4681
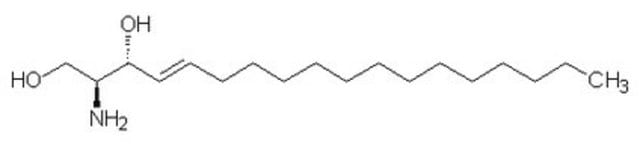
L-threo-Dihydrosphingosine
≥95% (TLC)
Synonyme(s) :
Safingol
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule empirique (notation de Hill):
C18H39NO2
Numéro CAS:
Poids moléculaire :
301.51
Numéro MDL:
Code UNSPSC :
12352211
ID de substance PubChem :
Nomenclature NACRES :
NA.77
Produits recommandés
Niveau de qualité
Essai
≥95% (TLC)
Forme
powder
Température de stockage
−20°C
Chaîne SMILES
CCCCCCCCCCCCCCC[C@H](O)[C@@H](N)CO
InChI
1S/C18H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(21)17(19)16-20/h17-18,20-21H,2-16,19H2,1H3/t17-,18-/m0/s1
Clé InChI
OTKJDMGTUTTYMP-ROUUACIJSA-N
Actions biochimiques/physiologiques
Sphingosine kinase inhibitor; protein kinase C alpha (PKCα) -specific inhibitor; Sphingosine analog; potentiates the effect of doxorubicin (DOX) in tumor-bearing animals.
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
J W Darges et al.
Advances in experimental medicine and biology, 400A, 387-392 (1997-01-01)
The sphingosine analog L-threo-dihydrosphingosine has been shown to inhibit protein kinase C (PKC) isoenzymes in mixed micelle and vesicle assays. This compound also inhibited the reactive oxygen intermediates (ROI) released from isolated neutrophils (IC50 approximately 2 microM) and phorbol ester-induced
G K Schwartz et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 3(4), 537-543 (1997-04-01)
We performed a pilot clinical trial with safingol (L-threo-dihydrosphingosine), a protein kinase C-specific inhibitor that potentiates the effect of doxorubicin (DOX) in tumor-bearing animals. Safingol was initially administered as a 1-h infusion at escalating doses. Fourteen days later, patients received
Yi-Hsin Hsu et al.
Cancer research, 74(17), 4822-4835 (2014-06-28)
Triple-negative breast cancer (TNBC) is a highly heterogeneous and recurrent subtype of breast cancer that lacks an effective targeted therapy. To identify candidate therapeutic targets, we profiled global gene expression in TNBC and breast tumor-initiating cells with a patient survival
Avraham Ashkenazi et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 26(11), 4628-4636 (2012-08-09)
Understanding the structural organization of lipids in the cell and viral membranes is essential for elucidating mechanisms of viral fusion that lead to entry of enveloped viruses into their host cells. The HIV lipidome shows a remarkable enrichment in dihydrosphingomyelin
Jesús Enrique Vizcarra-Olvera et al.
Mycopathologia, 174(3), 247-254 (2012-03-08)
This study was conducted to evaluate the possible protector effect of bentonite and zeolite in Bovans chicks fed a diet containing 59 mg kg(-1) of fumonisin B1 (FB1) during 3 weeks. A total of 200 one-day-old male chicks were treated
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique