C1261
Carboxypeptidase A−Agarose
ammonium sulfate suspension, ≥6 units/mL packed gel, 25 °C, enzyme from bovine pancreas
Synonyme(s) :
Carboxypolypeptidase, Peptidyl-L-amino-acid hydrolase
About This Item
Produits recommandés
Source biologique
enzyme from bovine pancreas
Niveau de qualité
Forme
ammonium sulfate suspension
Activité spécifique
≥6 units/mL packed gel, 25 °C
Poids mol.
~35,250
Matrice
beaded agarose
Température de stockage
2-8°C
Description générale
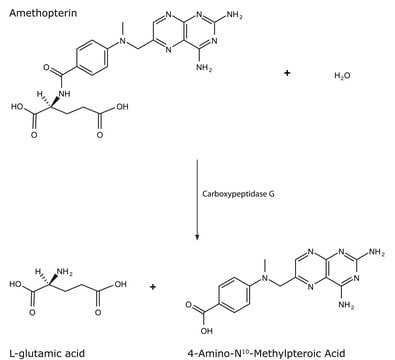
Actions biochimiques/physiologiques
Définition de l'unité
Forme physique
Inhibiteur
Substrat
Mention d'avertissement
Danger
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Listes réglementaires
Les listes réglementaires sont principalement fournies pour les produits chimiques. Seules des informations limitées peuvent être fournies ici pour les produits non chimiques. L'absence d'indication signifie qu'aucun des composants n'est répertorié. Il incombe à l'utilisateur de s'assurer de l'utilisation sûre et légale du produit.
EU REACH Annex XVII (Restriction List)
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique