11080725001
Roche
Neuraminidase (Sialidase)
from Vibrio cholerae
Synonyme(s) :
Salidase
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Produits recommandés
Source biologique
Vibrio cholerae
Niveau de qualité
Forme
solution
Poids mol.
~95 kDa
Conditionnement
pkg of 1 U
Fabricant/nom de marque
Roche
pH optimal
5.5-6.2
Adéquation
suitable for ELISA applications
Application(s)
life science and biopharma
sample preparation
Conditions d'expédition
wet ice
Température de stockage
2-8°C
Description générale
Approximately 40 U/mg enzyme protein at 37 °C and pH 5.5, with N-acetyl-neuraminosyl-D-lactose as the substrate.
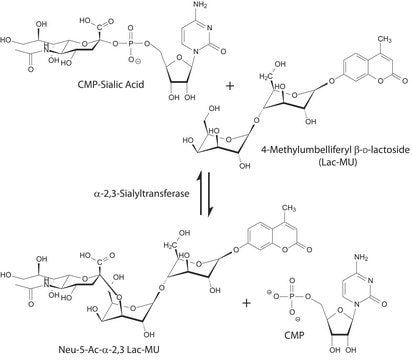
Neuraminidase is an acylneuraminyl hydrolase which hydrolyzes terminal N- or O-acylneuraminic acids which are α2,3-, α2,6-, or α2,8-linked (rate: α2,6 > α2,3 > α2,8) to oligosaccharides, polysaccharides, mucopolysaccharides, glycoproteins, and glycolipids. Noteworthy, for the hydrolysis of glycolipids, the presence of a detergent is necessary. Because of the broad substrate specificity, the enzyme is very well suited for the complete removal of sialic acids from glycoconjugates of a wide variety of biological materials (e.g., in cytology, on cell surfaces, viruses etc.).
Spécificité
Hydrolyzes terminal N- or O-acyl-neuraminic acids that are α2,3-, α2,6-, or α2,8-linked to galactose, Hex, NAc, or N- or O-acylated neuraminyl residues in oligosaccharides/glycoconjugates or colominic acid. Relative rate of cleavage is α2,3 >α2,6 >α2,8, determined on bonds in tri- and tetrasaccharides.
Application
Neuraminidase has been used:
- to remove cis-acting sialic acids in CHO (chinese hamster ovary) cells
- for deglycosylation studies
Définition de l'unité
One unit is the enzyme activity that hydrolyzes 1 μmol N-acetyl-neuraminosyl-D-lactose within 1 min at +37 °C under the following incubation conditions:
10 mM N-acetyl-neuraminosyl-D-lactose, 50 mM sodium acetate, 4 mM calcium chloride, bovine serum albumin, 100 μg/ml, pH 5.5. The activity is determined by measuring the released D-lactose using the β-galactosidase/galactose dehydrogenase method. Under the same conditions, 1 μmol N-acetylneuraminic acid per min is split off from human acid α1-glycoprotein (10 mg/ml incubation mixture) by 1 U neuraminidase. Released N-acetyl-neuraminic acid can be determined using, for example, the thiobarbituric acid method.
10 mM N-acetyl-neuraminosyl-D-lactose, 50 mM sodium acetate, 4 mM calcium chloride, bovine serum albumin, 100 μg/ml, pH 5.5. The activity is determined by measuring the released D-lactose using the β-galactosidase/galactose dehydrogenase method. Under the same conditions, 1 μmol N-acetylneuraminic acid per min is split off from human acid α1-glycoprotein (10 mg/ml incubation mixture) by 1 U neuraminidase. Released N-acetyl-neuraminic acid can be determined using, for example, the thiobarbituric acid method.
Forme physique
Solution in 50 mM sodium acetate, 154 mM sodium chloride, 9 mM calcium chloride, 0.1% Micr-O-Protect (w/v), human serum albumin, 25 mg/l, pH 5.5. The preparation contains 10 mM EDTA.
Note: The serum used for this preparation was tested for HBs antigen and for the presence of antibodies to HIV-1, HIV-2, HCV, and found to be negative, according to the current quality control procedures.
Note: The serum used for this preparation was tested for HBs antigen and for the presence of antibodies to HIV-1, HIV-2, HCV, and found to be negative, according to the current quality control procedures.
Autres remarques
For life science research only. Not for use in diagnostic procedures.
Informations légales
The sale of the Product does not exhaust or grant any rights in third party patents including patents of companies of the F. Hoffmann - La Roche AG group of companies, in particular, for the use of modified antibodies obtained by using the product.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Skin Sens. 1
Code de la classe de stockage
12 - Non Combustible Liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
does not flash
Point d'éclair (°C)
does not flash
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Direct Attachment with Erythrocytes Augments Extracellular Growth of Pathogenic Mycobacteria.
Nishiuchi, et al.
Microbiology spectrum, 10, e0245421-e0245421 (2022)
Angeles Canales et al.
JACS Au, 3(3), 868-878 (2023-04-04)
Influenza virus infection remains a threat to human health since viral hemagglutinins are constantly drifting, escaping infection and vaccine-induced antibody responses. Viral hemagglutinins from different viruses display variability in glycan recognition. In this context, recent H3N2 viruses have specificity for
Misako Nakayama et al.
Methods in molecular biology (Clifton, N.J.), 2556, 37-43 (2022-09-30)
Hemagglutinin (HA) on the surface of influenza viruses binds to sialic acids, mainly N-acetylneuraminic acid (Neu5Ac) or N-glycolylneuraminic acid. Neu5Ac and N-glycolylneuraminic acid lie at the terminal end of sugar chains on the cell surface. Human influenza viruses preferentially bind
A previously uncharacterized O-glycopeptidase from Akkermansia muciniphila requires the Tn-antigen for cleavage of the peptide bond.
Medley, et al.
The Journal of biological chemistry, 298, 102439-102439 (2023)
Peter L Delputte et al.
Journal of virology, 81(17), 9546-9550 (2007-06-15)
The sialic acid-binding lectin sialoadhesin (Sn) is a macrophage-restricted receptor for porcine reproductive and respiratory syndrome virus (PRRSV). To investigate the importance of pSn sialic acid-binding activity for PRRSV infection, an R(116)-to-E mutation was introduced in the predicted sialic acid-binding
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique