538396
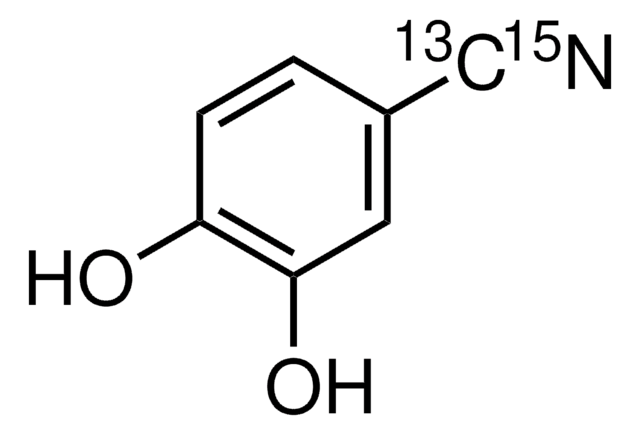
3,4-Dihydroxybenzonitrile
97%
Synonyme(s) :
Protocatechuonitrile
Se connecterpour consulter vos tarifs contractuels et ceux de votre entreprise/organisme
About This Item
Formule linéaire :
(OH)2C6H3CN
Numéro CAS:
Poids moléculaire :
135.12
Numéro CE :
Numéro MDL:
Code UNSPSC :
12352100
ID de substance PubChem :
Nomenclature NACRES :
NA.22
Produits recommandés
Niveau de qualité
Pureté
97%
Pf
155-159 °C (lit.)
Groupe fonctionnel
nitrile
Chaîne SMILES
Oc1ccc(cc1O)C#N
InChI
1S/C7H5NO2/c8-4-5-1-2-6(9)7(10)3-5/h1-3,9-10H
Clé InChI
NUWHYWYSMAPBHK-UHFFFAOYSA-N
Description générale
3,4-Dihydroxybenzonitrile can be prepared from 4-hydroxy-3-methoxybenzonitrile. It can also be synthesized by reacting 3,4-dimethoxybenzonitrile, lithium diisopropylamide (LDA) and 1,3-dimethyl-2-imidazolidinone (DMEU).
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
The synthetic technology of 3, 4-dihydroxybenzonitrile
WEI HW, et al.
Fine and Specialty Chemicals / Jing Xi Yu Zhuan Yong Hua Xue Pin, 9, 012-012 (2011)
Sodium Bis (trimethylsilyl) amide and Lithium Diisopropylamide in Deprotection of Alkyl Aryl Ethers: a-Effect of Silicon
Hwu JR, et al.
The Journal of Organic Chemistry, 62.12 , 4097-4104 (1997)
M J Nelson et al.
Biochemistry, 34(46), 15219-15229 (1995-11-21)
Ferric soybean lipoxygenase forms stable complexes with 4-substituted catechols. The structure of the complex between the enzyme and 3,4-dihydroxybenzonitrile has been studied by resonance Raman, electron paramagnetic resonance, visible, and X-ray spectroscopies. It is a bidentate iron-catecholate complex with at
M M Wick et al.
Journal of pharmaceutical sciences, 76(7), 513-515 (1987-07-01)
This report describes a structure-activity analysis of isomers of three classes of dihydroxybenzene derivatives, including dihydroxybenzaldoxime, dihydroxybenzaldehyde, and dihydroxybenzonitrile. These derivatives were examined for their effect on ribonucleotide reductase activity, macromolecular synthesis, cell growth, and in vivo antitumor activity against
The Reactivity and Reaction Pathway of Fenton Reactions Driven by Substituted 1,2-Dihydroxybenzenes.
Pablo Salgado et al.
Environmental science & technology, 51(7), 3687-3693 (2017-03-09)
Fenton systems are interesting alternatives to advanced oxidation processes (AOPs) applied in soil or water remediation. 1,2-Dihydroxybenzenes (1,2-DHBs) are able to amplify the reactivity of Fenton systems and have been extensively studied in biological systems and for AOP applications. To
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique