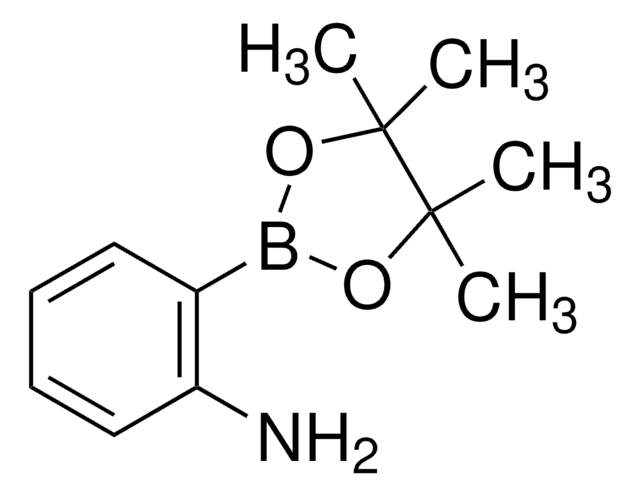
518751
4-Aminophenylboronic acid pinacol ester
97%
Synonyme(s) :
2-(4-Aminophenyl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)aniline, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzeneamine, 4-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)phenylamine, 4-Aminophenylboronic acid, pinacol cyclic ester
About This Item
Produits recommandés
Niveau de qualité
Pureté
97%
Pf
165-169 °C (lit.)
Chaîne SMILES
CC1(C)OB(OC1(C)C)c2ccc(N)cc2
InChI
1S/C12H18BNO2/c1-11(2)12(3,4)16-13(15-11)9-5-7-10(14)8-6-9/h5-8H,14H2,1-4H3
Clé InChI
ZANPJXNYBVVNSD-UHFFFAOYSA-N
Application
- The preparation of substituted 3-phenyl-4H-1-benzopyran-4-ones by reacting with iodochromones via Pd catalyzed Suzuki-Miyaura cross-coupling reaction.
- Mercury(II) detection by fluorometry with new fluorogenic indicators based on through-bond energy transfer from pentaquinone to rhodamine.
- Rhodium-catalyzed amination reactions.
- Palladium-catalyzed Suzuki cross-coupling to synthesize potential antitubercular and antimicrobial compounds.
It can also be used to prepare:
- Hexaphenylbenzene derivatives as a potential bioprobe and multichannel keypad system.
- Pyromellitic diimide-based polymer as matrix for solution-processable n-channel field-effect transistors.
- Alternating copolymers of oligoarylenes and naphthalene bisimides as low band-gap semiconductors with electrochemical and spectroelectrochemical behavior.
- γ-secretase modulators in the treatment of amyloid β formation.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique