409006
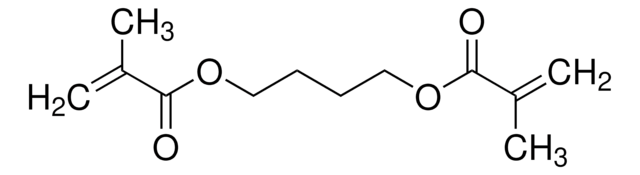
Di(ethylene glycol) dimethacrylate
95%, cross-linking reagent polymerization reactions, methacrylate, 300 ppm monomethyl ether hydroquinone as inhibitor
Synonyme(s) :
2,2′-Oxybisethanol dimethacrylate, 2,2′-Oxydiethyl dimethacrylate, Diethylene glycol, dimethacrylate, Polyethylene glycol
About This Item
Produits recommandés
product name
Di(ethylene glycol) dimethacrylate, 95%
Niveau de qualité
Pureté
95%
Forme
liquid
Contient
300 ppm monomethyl ether hydroquinone as inhibitor
Pertinence de la réaction
reagent type: cross-linking reagent
reaction type: Polymerization Reactions
Indice de réfraction
n20/D 1.458 (lit.)
Point d'ébullition
134 °C/2 mmHg (lit.)
Densité
1.082 g/mL at 25 °C (lit.)
Extrémité Ω
methacrylate
Extrémité α
methacrylate
Architecture des polymères
shape: linear
functionality: homobifunctional
Chaîne SMILES
CC(=C)C(=O)OCCOCCOC(=O)C(C)=C
InChI
1S/C12H18O5/c1-9(2)11(13)16-7-5-15-6-8-17-12(14)10(3)4/h1,3,5-8H2,2,4H3
Clé InChI
XFCMNSHQOZQILR-UHFFFAOYSA-N
Application
- Urethane dimethacrylate-based photopolymerizable resins for stereolithography 3D printing: A physicochemical characterisation and biocompatibility evaluation.: This study explores the use of urethane dimethacrylate-based resins in stereolithography 3D printing. It includes an in-depth physicochemical characterization and assesses the biocompatibility of the materials, highlighting their potential for medical and dental applications (Pitzanti G et al., 2024).
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique