125318
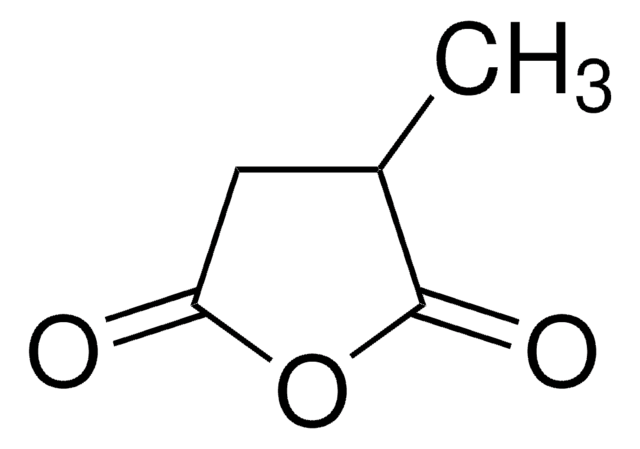
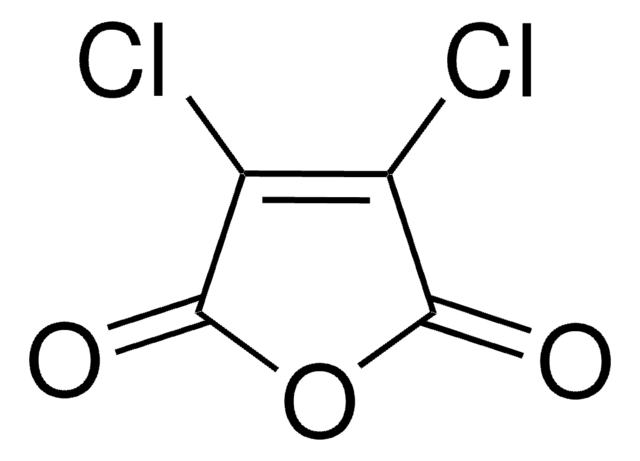
Citraconic anhydride
98%
Synonyme(s) :
2-Methylmaleic anhydride, 3-Methyl-2,5-furandione, Citraconic acid anhydride, Methylmaleic anhydride, Monomethylmaleic anhydride
About This Item
Produits recommandés
Densité de vapeur
4 (vs air)
Niveau de qualité
Pureté
98%
Forme
liquid
Indice de réfraction
n20/D 1.471 (lit.)
Point d'ébullition
213-214 °C (lit.)
Pf
6-10 °C (lit.)
Densité
1.247 g/mL at 25 °C (lit.)
Chaîne SMILES
CC1=CC(=O)OC1=O
InChI
1S/C5H4O3/c1-3-2-4(6)8-5(3)7/h2H,1H3
Clé InChI
AYKYXWQEBUNJCN-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- As an electrolyte additive for high-temperature pouch lithium-ion batteries. Citraconic anhydride reduces the interfacial impedance of pouch cells during high-temperature storage and enhances their stability.
- As a pH-sensitive linker to surface functionalization of biomolecules used in drug delivery systems. The high pH sensitivity of citraconic anhydride conjugates is attributed to the presence of a double bond that restricts the separation between the amide and carboxylic acid groups.
- As a reagent to synthesize new thiopyrano[2,3-d][1,3]thiazole derivatives via hetero-Diels–Alder reactions. These thiopyrano derivatives exhibit diverse biological activities such as anticancer, antiviral, and antitrypanosomal.
- As a co-monomer in the ring-opening polymerization with d-xylose 3,5-anhydrosugar derivative to form novel sugar-derived polyesters, with up to 100% renewable content. This can serve as a sustainable feedstock for polymer synthesis.
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Code de la classe de stockage
8A - Combustible corrosive hazardous materials
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
231.8 °F - closed cup
Point d'éclair (°C)
111 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique