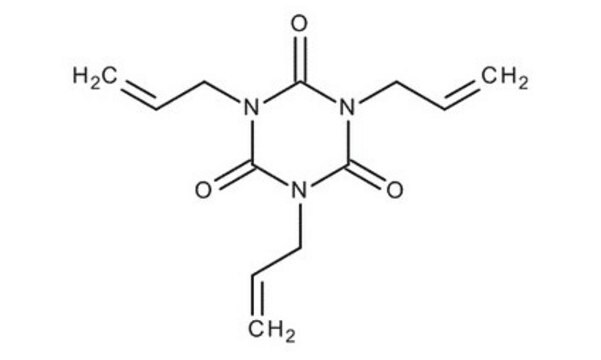
114235
1,3,5-Triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione
98%
Synonyme(s) :
1,3,5-Tri-2-propen-1-yl-1,3,5-triazine-2,4,6(1H ,3H ,5H )-trione, 1,3,5-Triallyl-1,3,5-triazinane-2,4,6-trione, 1,3,5-Triallylisocyanurate, 1,3,5-Triallylisocyanuric acid, 1,3,5-Tris-2′-propenylisocyanuric acid, Triallyl isocyanurate
About This Item
Produits recommandés
Niveau de qualité
Pureté
98%
Contient
500 ppm tert-butylhydroquinone as inhibitor
Indice de réfraction
n20/D 1.513 (lit.)
Point d'ébullition
149-152 °C/4 mmHg (lit.)
Densité
1.159 g/mL at 25 °C (lit.)
Chaîne SMILES
C=CCN1C(=O)N(CC=C)C(=O)N(CC=C)C1=O
InChI
1S/C12H15N3O3/c1-4-7-13-10(16)14(8-5-2)12(18)15(9-6-3)11(13)17/h4-6H,1-3,7-9H2
Clé InChI
KOMNUTZXSVSERR-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- A crosslinking agent or additive in the synthesis of polylactic acid /flax composite materials to enhance their properties and performance under gamma irradiation.
- A monomer in the synthesis of flexible ionogels with good mechanical properties via in situ thiol-ene photopolymerization with trimethylolpropane tris(3-mercaptopropionate). These ionogels further find applications in electrochemical capacitors.
- A monomer in the fabrication of the shape memory polymer substrates.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - STOT RE 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 1
Point d'éclair (°F)
319.1 °F - closed cup
Point d'éclair (°C)
159.5 °C - closed cup
Équipement de protection individuelle
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique


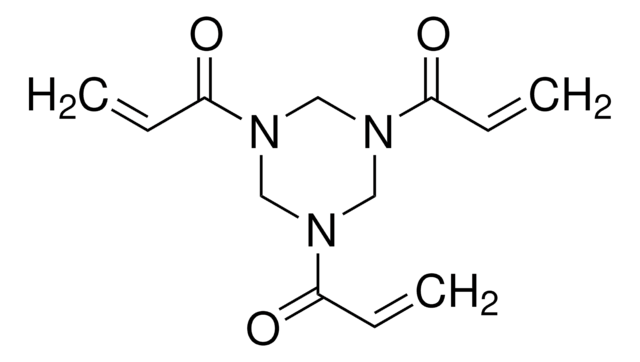

![Tris[2-(acryloyloxy)ethyl] isocyanurate](/deepweb/assets/sigmaaldrich/product/structures/254/494/1a620abc-8043-457f-92ec-87a959682438/640/1a620abc-8043-457f-92ec-87a959682438.png)





![Tricyclo[5.2.1.02,6]decanedimethanol diacrylate](/deepweb/assets/sigmaaldrich/product/structures/327/536/0dc81542-b920-47ec-99c1-d064a327a315/640/0dc81542-b920-47ec-99c1-d064a327a315.png)
![Tris[3-(trimethoxysilyl)propyl] isocyanurate technical grade](/deepweb/assets/sigmaaldrich/product/structures/239/690/c24b2d6d-4580-41dd-a3ec-77f7fcb9caaf/640/c24b2d6d-4580-41dd-a3ec-77f7fcb9caaf.png)