103276
Harmane
98%
Synonyme(s) :
1-Methyl-9H-pyrido[3,4-b]indole, 2-Methyl-β-carboline, Aribine
About This Item
Produits recommandés
Niveau de qualité
Pureté
98%
drug control
stupéfiant (France)
Pf
235-238 °C (lit.)
Solubilité
methanol: soluble 50mg/ml
Chaîne SMILES
Cc1nccc2c3ccccc3[nH]c12
InChI
1S/C12H10N2/c1-8-12-10(6-7-13-8)9-4-2-3-5-11(9)14-12/h2-7,14H,1H3
Clé InChI
PSFDQSOCUJVVGF-UHFFFAOYSA-N
Informations sur le gène
human ... CYP2D6(1565)
rat ... Gabra2(29706)
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
- Harmane is a potent tremor-producing β-carboline alkaloid and neurotoxin.
- It is major representative of heterocyclic aromatic amines, a group of mutagenic and carcinogenic substances which are formed in meat from the precursors creatine, creatinine, amino acids and sugars during the heating at high temperatures.
- Blood harmane concentration is elevated in essential tremor, late-life neurological disease.
Application
- Harmane was used in trace level determination of harmane by planar chromatography coupled with (tandem) mass spectrometry.
- It was used to study interactions of norharman and harman with DNA.
- It may be used as matrix for analysis of cyclodextrins and for sulfated oligosaccharides in combination with DHB as co-matrix.
Actions biochimiques/physiologiques
Notes préparatoires
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Acute Tox. 4 Oral - Skin Irrit. 2
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique
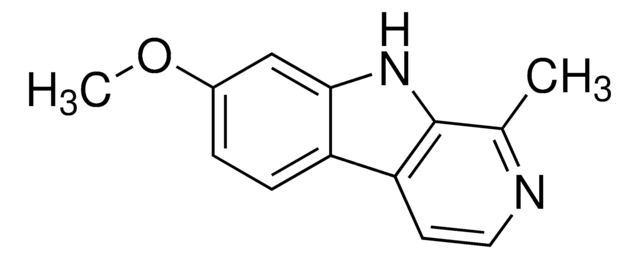

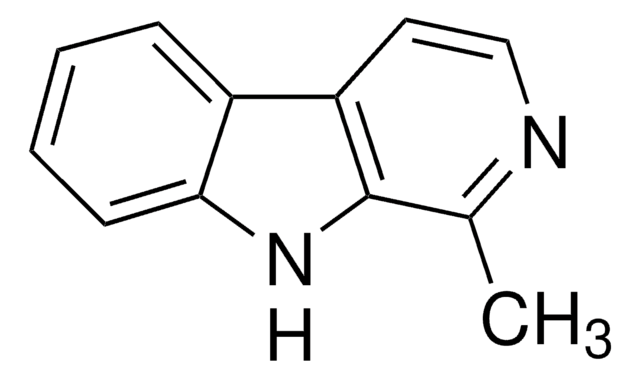
![1,2,3,4-Tetrahydro-9H-pyrido[3,4-b]indole 98%](/deepweb/assets/sigmaaldrich/product/structures/181/460/3d58bc34-1b5c-4295-bbac-3b52085670e8/640/3d58bc34-1b5c-4295-bbac-3b52085670e8.png)
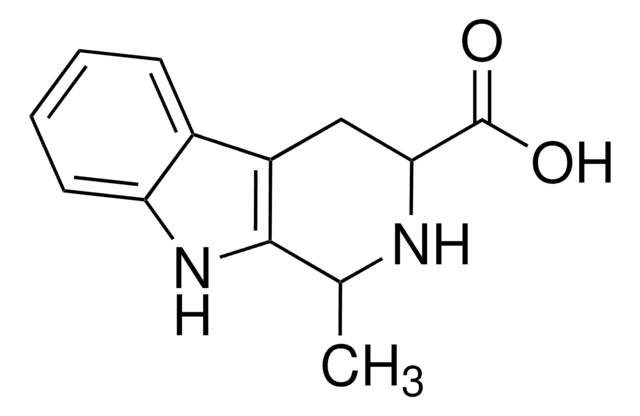
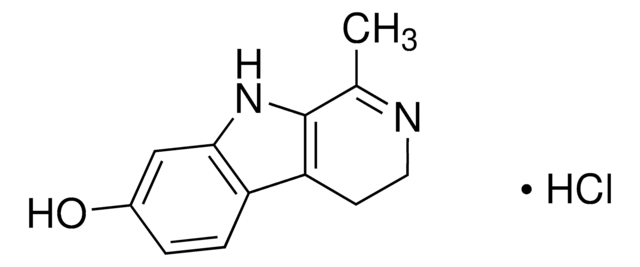
![9H-pyrido[2,3-b]indole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/418/992/8c7bac06-11e8-45d7-b863-5d35b582e871/640/8c7bac06-11e8-45d7-b863-5d35b582e871.png)

![2,3,4,5-Tetrahydro-1H-pyrido[4,3-b]indole AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/376/664/07577eb6-6e8c-4237-b8c5-03da4c8e7d88/640/07577eb6-6e8c-4237-b8c5-03da4c8e7d88.png)
