05091
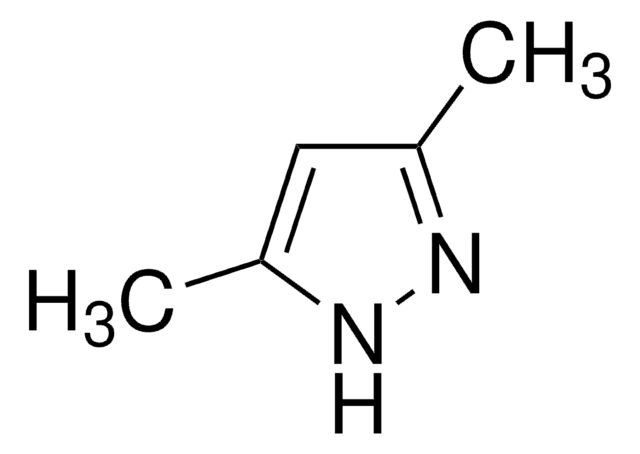
3,5-Dimethylpyrazole
produced by Wacker Chemie AG, Burghausen, Germany, ≥99.0% (GC)
Synonym(s):
3,5-Dimethyl-1H-pyrazole
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8N2
CAS Number:
Molecular Weight:
96.13
Beilstein:
106325
EC Number:
MDL number:
UNSPSC Code:
12352100
eCl@ss:
39160503
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
produced by Wacker Chemie AG, Burghausen, Germany
Quality Level
Assay
≥99.0% (GC)
bp
218 °C (lit.)
mp
105-108 °C (lit.)
SMILES string
Cc1cc(C)[nH]n1
InChI
1S/C5H8N2/c1-4-3-5(2)7-6-4/h3H,1-2H3,(H,6,7)
InChI key
SDXAWLJRERMRKF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - STOT RE 2
Target Organs
Liver
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Sara Straniero et al.
Rejuvenation research, 12(2), 77-84 (2009-05-08)
Aging is characterized by several metabolic changes responsible for the decline of certain functions and the appearance of age-related diseases, including hypercholesterolemia, which is the main risk factor for atherosclerosis and cardiovascular disease. Similar changes in a number of morphological
Rupam Sarma et al.
Dalton transactions (Cambridge, England : 2003), (36)(36), 7428-7436 (2009-09-04)
The reactions of 3,5-dimethylpyrazole with zinc(II)acetate dihydrate and varieties of aromatic carboxylic acids led to formation of mono-nuclear zinc complexes of composition [Zn(HDMP)2(RCO2)2] (R = C6H5, p-CH3-C6H4, p-NO2-C6H4 etc. HDMP = 3,5-dimethylpyrazole) in methanol, whereas the same reactants in dimethylformamide
J M Orza et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 56A(8), 1469-1498 (2000-07-25)
The infrared (IR) and Raman spectra of 3,5-dimethylpyrazole have been recorded in the vapor, liquid (melt and solution) and solid states. Two deuterated derivatives, C5H7N-ND and C5D7N-NH, were also studied in solid state and in solutions. Instrumental resolution was relatively
T Locci Cubeddu et al.
Biochimica et biophysica acta, 839(1), 96-104 (1985-03-29)
The mechanisms involved in the inhibitory effects of antilipolytic agents on rat liver peroxisomal fatty acid oxidative activity have been explored. Treatment of fasting rats with antilipolytic drugs (either 3,5-dimethylpyrazole (12 mg/kg body weight) or Acipimox (25 mg/kg body weight]
C Redekopp et al.
Journal of endocrinological investigation, 3(3), 237-241 (1980-07-01)
To investigate the suppressive effect of somatostatin on growth hormone secretion, a consistent, potent stimulus to growth hormone release is required. The antilipolytic compound 3,5-dimethylpyrazole (DMP) gave a rapid rise in plasma immunoreactive growth hormone following iv administration to fasting
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service