8.56044
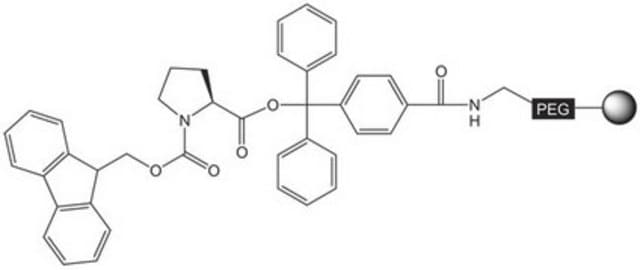
Fmoc-Cys(Trt)-NovaSyn® TGT
for peptide synthesis, Novabiochem®
Synonym(s):
Trityl-Protected Cysteine Resin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352209
NACRES:
NA.22
Recommended Products
product name
Fmoc-Cys(Trt)-NovaSyn® TGT, Novabiochem®
Quality Level
product line
NovaSyn® TG
Novabiochem®
form
solid
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
15-25°C
General description
Pre-loaded resin for synthesis of peptide acids and protected peptide fragments containing a C-terminal cysteine amino-acid residue by Fmoc SPPS. The base NovaSyn® TG is a composite of low cross-linked polystyrene and 3000-4000 M.W. polyethylene glycol. Peptide synthesis is carried out at the ends of the PEG chains that have been functionalized with the hyper-acid labile 4-carboxytrityl alcohol linker. This use of this linker helps prevent racemization and β-piperidinylalanine formation during chain extension.Treatment of the peptidyl resin with 20% TFE in DCM or 1% TFA in DCM cleaves the product from the resin without affecting the standard TFA-labile side-chain protecting groups. Standard TFA cleavage releases the fully deprotected peptide.
Associated Protocols and Technical Articles:
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references:
[1] E. Atherton, et al. in "Peptides 1990, Proc. 21st European Peptide Symposium, E. Giralt & D. Andreu(Eds), 1991, Escom, Leiden, pp. 243
[2] J. Lukszo, et al. (1996) Lett. Pept. Sci., 3, 157.
[3] Y. Fujiwara, et al. (1994) Chem. Pharm. Bull., 42, 724.
[4] K. Barlos & D. Gatos in “Fmoc solid phase peptide synthesis: a practical approach”, W. C. Chan & P. D.White (Eds.), Oxford University Press, Oxford, 2000, pp. 218.
Associated Protocols and Technical Articles:
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references:
[1] E. Atherton, et al. in "Peptides 1990, Proc. 21st European Peptide Symposium, E. Giralt & D. Andreu(Eds), 1991, Escom, Leiden, pp. 243
[2] J. Lukszo, et al. (1996) Lett. Pept. Sci., 3, 157.
[3] Y. Fujiwara, et al. (1994) Chem. Pharm. Bull., 42, 724.
[4] K. Barlos & D. Gatos in “Fmoc solid phase peptide synthesis: a practical approach”, W. C. Chan & P. D.White (Eds.), Oxford University Press, Oxford, 2000, pp. 218.
Application
Applications of Fmoc-Cys(Trt)-NovaSyn® TGT include:
- the preparation of a phosphoprotein using Native Chemical Ligation (NCL).
- the preparation of a peptide intermediate for use in glycoprotein semi-synthesis.
Linkage
Replaces: 04-12-2705
Legal Information
NOVASYN is a registered trademark of Merck KGaA, Darmstadt, Germany
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Access to phosphoproteins and glycoproteins through semi-synthesis, Native Chemical Ligation and N? S acyl transfer
Masania J, et al.
Organic & Biomolecular Chemistry, 5113-5119 (2010)
Racemization-free synthesis of C-terminal cysteine-peptide using 2-chlorotrityl resin
Y. Fujiwara, et al.
Journal of Chemical and Pharmaceutical Sciences
, 42, 724-724 (1994)
3-(1-Piperidinyl) alanine formation during the preparation of C-terminal cysteine peptides with the Fmoc/t-Bu strategy
J. Lukszo, et al.
Peptide science (Hoboken, N.J.), 3, 157-157 (1996)
K. Barlos & D. Gatos in ?Fmoc solid phase peptide synthesis: a practical approach?, W. C. Chan & P. D.
White (Eds.), Oxford University Press, Oxford
K. Barlos & D. Gatos
Solid Phase Peptide Synthesis: A Practical Approach, 218-218 (2000)
Peptides 1990, Proc. 21st European Peptide Symposium, E. Giralt & D. Andreu
E. Atherton, et al. in
Proceedings, 243-243 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service