M72609
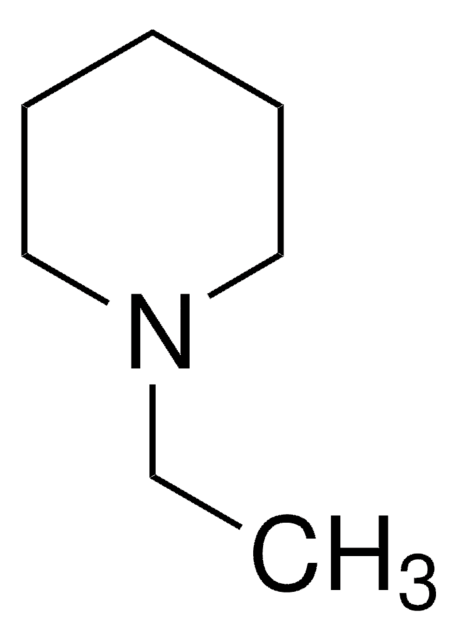
N-Methylpiperidine
99%
Synonym(s):
1-Methylpiperidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H13N
CAS Number:
Molecular Weight:
99.17
Beilstein:
1073
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.4378 (lit.)
bp
106-107 °C (lit.)
density
0.816 g/mL at 25 °C (lit.)
SMILES string
CN1CCCCC1
InChI
1S/C6H13N/c1-7-5-3-2-4-6-7/h2-6H2,1H3
InChI key
PAMIQIKDUOTOBW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Reactant for:
Reactant for synthesis of:
- sp3 C-H Bond activation with ruthenium(II) catalysts and C(3)-alkylation of cyclic amines
- One-pot synthesis of Z-cinnamic acids
Reactant for synthesis of:
- Unsymmetrical ureas
- Antibacterial imidazolium, pyrrolidinium, and piperidinium salts
- C1-C16 segment of goniodomin A via palladium-catalyzed organostannane thioester coupling
- Multi-targeted inhibitors of insulin-like growth factor-1 receptor and members of ErbB-family receptor kinases
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
37.4 °F - closed cup
Flash Point(C)
3 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Oscar B Torres et al.
Analytical and bioanalytical chemistry, 410(16), 3885-3903 (2018-04-21)
We describe for the first time a method that utilizes microscale thermophoresis (MST) technology to determine polyclonal antibody affinities to small molecules. Using a novel type of heterologous MST, we have accurately measured a solution-based binding affinity of serum antibodies
Xuenan Li et al.
Journal of food science, 85(9), 2754-2761 (2020-08-15)
N,N-dimethylpiperidinium (mepiquat) is a new process-induced compound formed from natural constituents during the cooking process. Mepiquat was first found in coffee and cereal products, but its formation mechanism in coffee is still unclear. In the current study, Arabica and Robusta
Francisc Potmischil et al.
Magnetic resonance in chemistry : MRC, 45(3), 231-235 (2007-01-16)
The (15)N chemical shifts of 13 N-methylpiperidine-derived mono-, bi- and tricycloaliphatic tertiary amines, their methiodides and their N-epimeric pairs of N-oxides were measured, and the contributions of specific structural parameters to the chemical shifts were determined by multilinear regression analysis.
A Kolocouris et al.
The Journal of organic chemistry, 66(15), 4989-4997 (2001-07-21)
When a 1-adamantyl or a 2-adamantyl substituent is introduced at the 2-position in N-methylpiperidine, four different chair conformations are possible. Experimental observation using dynamic NMR spectroscopy and molecular mechanics calculations agree that the chair conformation with an equatorial adamantyl group
[The mechanism of reverse inhibition of cholinesterases by thionphosphonates].
N N Kovalev et al.
Izvestiia Akademii nauk SSSR. Seriia biologicheskaia, (6)(6), 926-929 (1988-11-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service