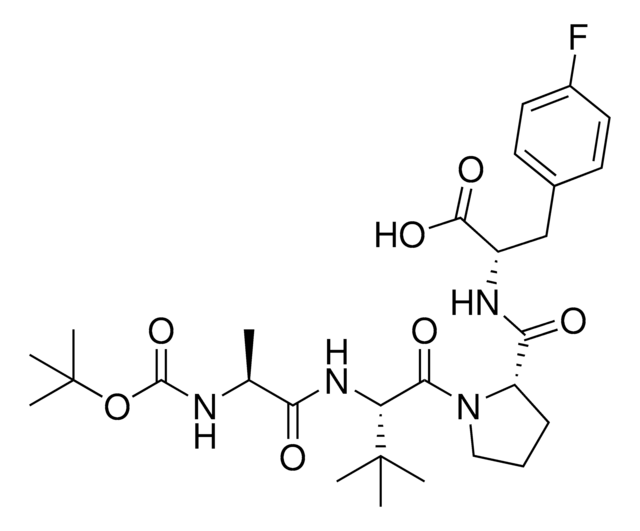
916714
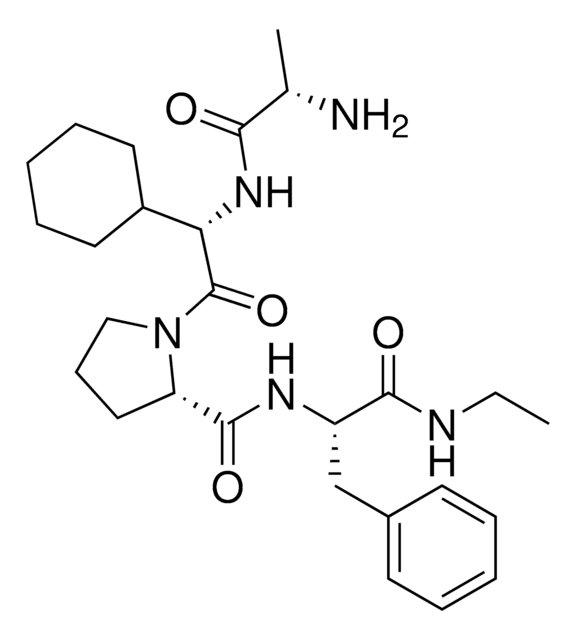
A1V2PF2-NHEt
≥95%
Synonym(s):
(S)-1-((S)-2-((S)-2-Aminopropanamido)-2-cyclohexylacetyl)-N-((S)-1-(ethylamino)-3-(4-fluorophenyl)-1-oxopropan-2-yl)pyrrolidine-2-carboxamide, AVP ligand, IAP E3 ligase lead for protein degrader research, SNIPER building block
About This Item
Recommended Products
ligand
A1V2PF2
Quality Level
Assay
≥95%
form
powder
reaction suitability
reagent type: ligand
functional group
amine
storage temp.
2-8°C
SMILES string
N[C@H](C(N[C@H](C(N1CCC[C@H]1C(N[C@H](C(NCC)=O)CC2=CC=C(C=C2)F)=O)=O)C3CCCCC3)=O)C
Related Categories
Application
A1V2PF2-NHEt conjugates are also available for degrader synthesis. Browse our full synthesis offering here for streamlining SNIPER and PROTAC® degrader libraries: Degrader Building Blocks with Inhibitor of Apoptosis Protein (IAP) In Silico-Derived Ligands
917931 A1V2PF2-NHEt-C6-NH2
916684 A1V2PF2-NHEt-C10-NH2
916935 A1V2PF2-NHEt-PEG1-NH2
917192 A1V2PF2-NHEt-PEG3-NH2
Other Notes
In Vivo Knockdown of Pathogenic Proteins via Specific and Nongenetic Inhibitor of Apoptosis Protein (IAP)-dependent Protein Erasers (SNIPERs)
SNIPERs−Hijacking IAP activity to induce protein degradation
E3 Ligase Ligands for PROTACs: How They Were Found and How to Discover New Ones
Legal Information
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service