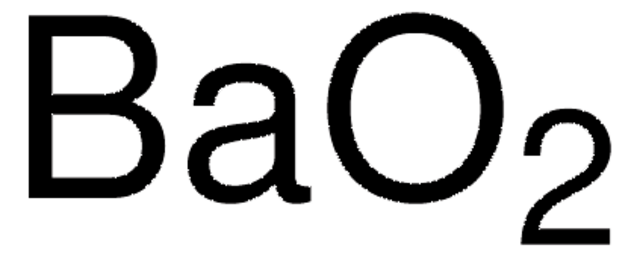72262
Nickel (IV) oxide
technical, oxidizing agent, ~30% active peroxide basis
Synonym(s):
Nickel dioxide, Nickel oxide
About This Item
Recommended Products
grade
oxidizing agent
technical
Quality Level
form
powder
reaction suitability
core: nickel
reagent type: catalyst
reagent type: oxidant
concentration
~30% (active peroxide)
SMILES string
O=[Ni]O[Ni]=O
InChI
1S/2Ni.3O
InChI key
PZFKDUMHDHEBLD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- One-dimensional oxide-metal hybrid structures and site-specific enhanced reactivity for CO oxidation
- Microwave induced catalytic degradation of crystal violet
Used to study differences in reactivity trends for carbon monoxide catalyzed oxidation caused by anionic transition metal oxide clusters
Reagent:
- For solvent-free oxidation of alcohols†
- For chemoselective oxidation of organic compounds using inductive heating
- To improve the synthesis of methoxy cephalosporin′s intermediate
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 4 - Carc. 1A Inhalation - Ox. Sol. 2 - Skin Sens. 1 - STOT RE 1 Inhalation
Storage Class Code
5.1B - Oxidizing hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service