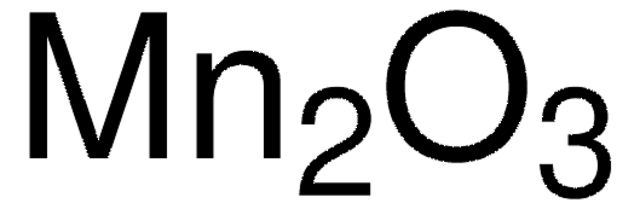377473
Manganese(II,III) oxide
97%
Synonym(s):
MnO.Mn2O3
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Mn3O4
CAS Number:
Molecular Weight:
228.81
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
powder
density
4.8 g/mL at 25 °C (lit.)
application(s)
battery manufacturing
SMILES string
O=[Mn]O[Mn]O[Mn]=O
InChI
1S/3Mn.4O
InChI key
GVNFAUMGUISVJW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Manganese(II,III) oxide is a transition metal oxide that is formed by annealing manganese oxide in the air above 1000°C. It can be used for a variety of applications such as catalysis, electrochromic devices, and other energy storage applications.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Chemistry of elements (2012)
Lucas K Wagner
The Journal of chemical physics, 138(9), 094106-094106 (2013-03-15)
Very accurate wave functions are calculated for small transition metal oxide molecules. These wave functions are decomposed using reduced density matrices to study the underlying correlation of electrons. The correlation is primarily of left-right type between the transition metals and
Y Zhang et al.
Journal of hazardous materials, 248-249, 81-88 (2013-01-23)
Several MCM-41 materials were synthesized at different conditions by hydrothermal procedure using cheap and easily available industrial water glass as silica source. Fe doped manganese-based oxide/MCM-41 sorbents were prepared by a sol-gel method. The effects of loadings of metal oxide
Nancy Birkner et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(22), 8801-8806 (2013-05-15)
Previous measurements show that calcium manganese oxide nanoparticles are better water oxidation catalysts than binary manganese oxides (Mn3O4, Mn2O3, and MnO2). The probable reasons for such enhancement involve a combination of factors: The calcium manganese oxide materials have a layered
Andres M Cardenas-Valencia et al.
Rapid communications in mass spectrometry : RCM, 27(5), 635-642 (2013-02-16)
In situ analytical techniques that require the storage and delivery of reagents (e.g., acidic or basic solutions) have inherent durability limitations. The reagentless electrolytic technique for pH modification presented here was developed primarily to ease and to extend the longevity
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service