210145
2,2-Dimethyl-1,3-dioxane-4,6-dione
98%
Synonym(s):
Malonic acid cyclic isopropylidene ester, Meldrum’s acid, cycl-Isopropylidene malonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8O4
CAS Number:
Molecular Weight:
144.13
Beilstein:
117310
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
92-96 °C (lit.)
solubility
dioxane: soluble 5%, clear to very slightly hazy, colorless to faintly yellow
functional group
ester
ketal
storage temp.
2-8°C
SMILES string
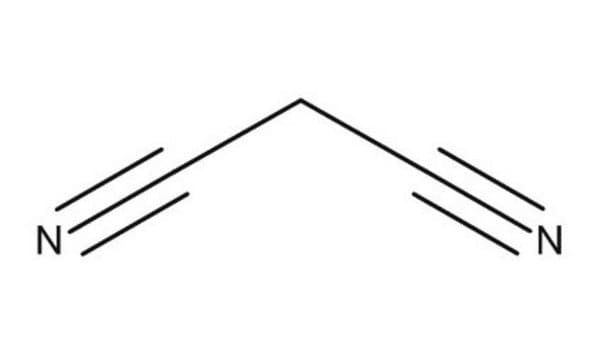
CC1(C)OC(=O)CC(=O)O1
InChI
1S/C6H8O4/c1-6(2)9-4(7)3-5(8)10-6/h3H2,1-2H3
InChI key
GXHFUVWIGNLZSC-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2,2-Dimethyl-1,3-dioxane-4,6-dione (Meldrum′s acid) is widely used in organic synthesis, especially for multiple C-C bond formations due to its adequate acidity (pKa 4.83) and steric rigidity. Knoevenagel condensation reaction between aldehydes and Meldrum′s acid are accelerated in ionic liquids.
Meldrum′s acid is used as a valuable starting material to synthesize heterocycles and as intermediates in organic synthesis reactions.
Meldrum′s acid is used as a valuable starting material to synthesize heterocycles and as intermediates in organic synthesis reactions.
Application
2,2-Dimethyl-1,3-dioxane-4,6-dione was used in the synthesis of:
- macrocyclic β-keto lactone
- 4-pyridyl-substituted heterocycles
- 2-substituted indoles
- isofraxidin.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
New multicomponent domino reactions (MDRs) in water: highly chemo-, regio-and stereoselective synthesis of spiro {[1, 3] dioxanopyridine}-4, 6-diones and pyrazolo [3, 4-b] pyridines.
Ma N, et al.
Green Chemistry, 12?(8), 1357-1361 (2010)
Songlei Zhu et al.
Molecules (Basel, Switzerland), 17(12), 13856-13863 (2012-11-24)
A series of 4-aryl-6-methyl-3,4-dihydro-2H-pyrano[3,2-c]quinolin-2,5(6H)-diones were synthesized via the three-component reactions of aromatic aldehydes, 4-hydroxy-1-methylquinolin-2(1H)-one, and Meldrum's acid catalyzed by L-proline. The structures of the products were identified by spectroscopic analysis. A mechanism for this three-component reaction catalyzed by L-proline was
Davood Nematollahi et al.
Chemical & pharmaceutical bulletin, 58(1), 23-26 (2010-01-05)
Electrochemical oxidation of catechols in the presence of phenyl-Meldrum's acid as a nucleophile in aqueous solution has been studied in detail by means of cyclic voltammetry and controlled potential coulometry. The results indicate that the o-benzoquinone derived from catechols participates
The synthesis of β-keto lactones via cyclization of β-keto ester dianions or the cyclization of Meldrum's acid derivatives.
Lermer L, et al.
Canadian Journal of Chemistry, 70(5), 1427-1445 (1992)
Characterization of Meldrum?s acid derivative 5-(5-Ethyl-1, 3, 4-thiadiazol-2-ylamino) methylene-2, 2-dimethyl-1, 3-dioxane-4, 6-dione by Raman and FT-IR spectroscopy and DFT calculations
De Toledo TA, et al.
Journal of Molecular Structure, 37-42, 1091-1091 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service