All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H3N3O2
CAS Number:
Molecular Weight:
113.07
Beilstein:
2815
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
mp
303 °C (dec.) (lit.)
SMILES string
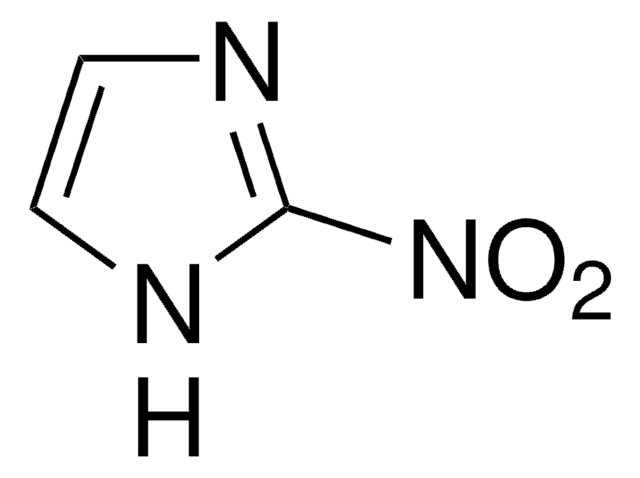
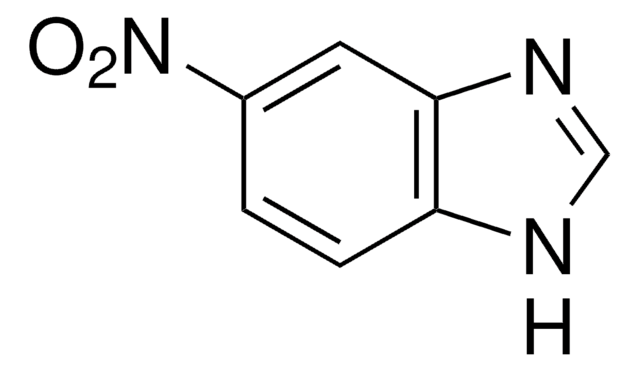
[O-][N+](=O)c1c[nH]cn1
InChI
1S/C3H3N3O2/c7-6(8)3-1-4-2-5-3/h1-2H,(H,4,5)
InChI key
VYDWQPKRHOGLPA-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Nitroimidazole is an intermediate during the synthesis of 1-methyl-2,4,5-trinitro imidazole.
Application
4-Nitroimidazole was used in a study to investigate the catalytic efficiency of heterocyclic compounds in the peroxyoxalate chemiluminescence reaction using bis(2,4,6-trichlorophenyl)oxalate as reagent.
related product
Product No.
Description
Pricing
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
392.0 °F - closed cup
Flash Point(C)
200 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Heterocyclic compounds as catalysts in the peroxyoxalate chemiluminescence reaction of bis (2, 4, 6-trichlorophenyl) oxalate.
Jonsson T and Irgum K.
Analytica Chimica Acta, 361(3), 205-215 (1998)
Quantum-chemical studies on thermodynamic feasibility of 1-methyl-2,4,5-trinitroimidazole processes.
Pandurang M Jadhav et al.
Journal of molecular modeling, 19(8), 3027-3033 (2013-04-12)
1-Methyl-2,4,5-trinitro imidazole (MTNI) is a well-known melt cast explosive possessing good thermal stability and impact insensitivity. MTNI has been synthesized from multi-step nitration followed by methylation of imidazole exhibiting low yield. It is desirable to screen the process thermodynamically for
David Leitsch et al.
The Journal of antimicrobial chemotherapy, 66(8), 1756-1765 (2011-05-24)
The mechanism of action of, and resistance to, metronidazole in the anaerobic (or micro-aerotolerant) protozoan parasite Giardia lamblia has long been associated with the reduction of ferredoxin (Fd) by the enzyme pyruvate:ferredoxin oxidoreductase (PFOR) and the subsequent activation of metronidazole
Raúl Argüello-García et al.
Infection, genetics and evolution : journal of molecular epidemiology and evolutionary genetics in infectious diseases, 9(6), 1057-1064 (2009-06-02)
The susceptibility of Giardia duodenalis trophozoites exposed in vitro to sublethal concentrations of metronidazole (MTZ) and albendazole (ABZ) may exhibit inter-culture (variability) and intra-culture (variation) differences in drug susceptibility. It was previously reported that MTZ-resistant trophozoites may display changes in
Carlos A Valdez et al.
Journal of medicinal chemistry, 52(13), 4038-4053 (2009-06-02)
Infections with the diarrheagenic pathogen, Giardia lamblia, are commonly treated with the 5-nitroimidazole (5-NI) metronidazole (Mz), and yet treatment failures and Mz resistance occur. Using a panel of new 2-ethenyl and 2-ethanyl 5-NI derivatives, we found that compounds with a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service