Alle Fotos(3)
Wichtige Dokumente
N17602
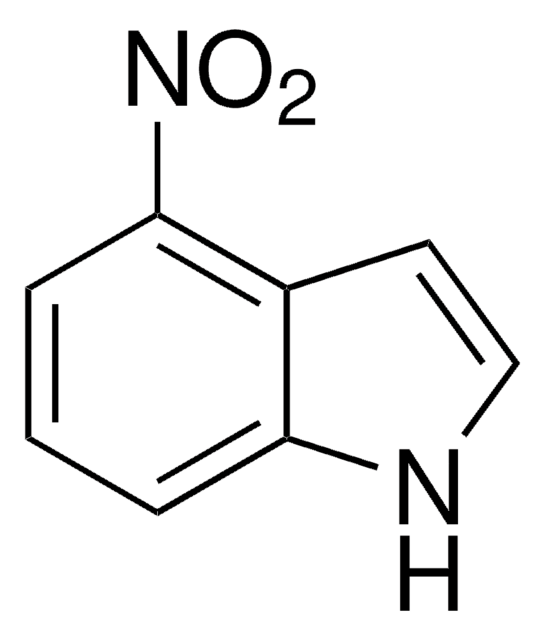
5-Nitroindol
98%
Synonym(e):
NSC 520594
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Empirische Formel (Hill-System):
C8H6N2O2
CAS-Nummer:
Molekulargewicht:
162.15
Beilstein:
383777
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
powder
mp (Schmelzpunkt)
140-142 °C (lit.)
SMILES String
[O-][N+](=O)c1ccc2[nH]ccc2c1
InChI
1S/C8H6N2O2/c11-10(12)7-1-2-8-6(5-7)3-4-9-8/h1-5,9H
InChIKey
OZFPSOBLQZPIAV-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Anwendung
Reactant for preparation of:
- Pharmaceutically active 2-oxo-1-pyrrolidine analogues
- Tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Protein Kinase Inhibitors and antiproliferative agents
- Positive Allosteric Modulators of Metabotropic Glutamate Receptor 4 (mGlu4)
- Antifungal agents
- Cannabinoid receptor type 1 (CB1) antagonists
- Potential anticancer agents
- Potential antivascular agents
- Selective Anti-leukemic agents
- Anti human immunodeficiency virus subtype 1 (HIV-1) agents
Signalwort
Danger
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral - Eye Dam. 1
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Caroline Crey-Desbiolles et al.
Nucleic acids research, 33(5), 1532-1543 (2005-03-16)
Universal DNA base analogs having photocleavable properties would be of great interest for development of new nucleic acid fragmentation tools. The photocleavable 7-nitroindole 2'-deoxyribonucleoside d(7-Ni) was previously shown to furnish a highly efficient approach to photochemically trigger DNA backbone cleavage
D Loakes et al.
Nucleic acids research, 23(13), 2361-2366 (1995-07-11)
3-Nitropyrrole and 5-nitroindole have been assessed as universal bases in primers for dideoxy DNA sequencing and in the polymerase chain reaction (PCR). In contrast to a previous report, we have found that the introduction of more than one 3-nitropyrrole residue
Junbin Zhang et al.
Chembiochem : a European journal of chemical biology, 13(13), 1940-1945 (2012-08-14)
During the formation of RNA-induced silencing complex (RISC), the passenger and guide strand of an siRNA duplex separate from each other to generate an active RISC complex. Accumulating evidence shows that an siRNA passenger strand can also assemble into a
S Ball et al.
Nucleic acids research, 26(22), 5225-5227 (1998-11-04)
Studies have been carried out on the use of octamer oligonucleotides tailed with different base analogues as primers in cycle sequencing reactions. 5-Nitroindole tails improved the performance as primers of a number of octamers. A tail length of three or
D Loakes et al.
Nucleic acids research, 22(20), 4039-4043 (1994-10-11)
4-, 5- and 6-Nitroindole have been investigated and compared with 3-nitropyrrole as universal bases in oligodeoxynucleotides. Of these the 5-nitroindole derivative was found to be superior giving higher duplex stability, and behaving indiscriminately towards each of the four natural bases
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.