Alle Fotos(1)
Wichtige Dokumente
M51407
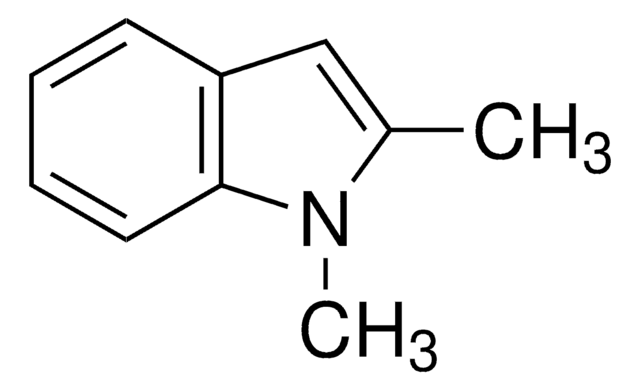
2-Methyl-indol
98%
Synonym(e):
NSC 7514
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C9H9N
CAS-Nummer:
Molekulargewicht:
131.17
Beilstein:
109781
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
bp
273 °C (lit.)
mp (Schmelzpunkt)
57-59 °C (lit.)
SMILES String
Cc1cc2ccccc2[nH]1
InChI
1S/C9H9N/c1-7-6-8-4-2-3-5-9(8)10-7/h2-6,10H,1H3
InChIKey
BHNHHSOHWZKFOX-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
Reactant for:
- Regioselective synthesis of oxopyrrolidine analogs via iodine-catalyzed Markovnikov addition reaction
- Friedel-Crafts alkylation reactions
- Preparation of tryptophan dioxygenase inhibitors pyridyl-ethenyl-indoles as potential anticancer immunomodulators
- Preparation of plant-growth inhibitors
- Michael addition reactions
- Synthesis of cyclooxygenase-1 (COX-1)/cyclooxygenase-2 (COX-2) inhibitors
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Acute Tox. 4 Oral
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
285.8 °F
Flammpunkt (°C)
141 °C
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
V F Ximenes et al.
Archives of biochemistry and biophysics, 387(2), 173-179 (2001-05-24)
The indole moeity is present in many substances of biological occurrence. Its metabolism, in most cases, involves an oxidative pathway. This study reports the oxidation of a series of indole derivatives, including several of biological origin, catalyzed by horseradish peroxidase
J M Gutteridge
The International journal of biochemistry, 14(7), 649-653 (1982-01-01)
1. The thiobarbituric acid (TBA) reaction, widely applied to the detection of autoxidation in polyunsaturated fatty acids, can be used to measure free-radical damage to amino acids, carbohydrates and nucleic acids. 2. In all of these systems malondialdehyde (MDA) is
Emma L Harry et al.
The Analyst, 136(8), 1728-1732 (2011-02-26)
The potential of ion mobility (IM) spectrometry in combination with mass spectrometry (MS) for real-time reaction monitoring is reported. The combined IM-MS approach using electrospray ionization affords gas-phase analyte characterization based on both mass-to-charge (m/z) ratio and gas-phase ion mobility
T Bhattacharya et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 60(8-9), 1957-1966 (2004-07-14)
Electrochemical measurements by cyclic voltammetry predict the possibility of occurrence of photoinduced electron-transfer (PET) reactions between the ground state of 2-phenylindole (2PI) (electron donor) and the excited singlet of 9-cyanoanthracene (9CNA) molecule acting as an electron acceptor. However, 2PI should
T Misra et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 58(8), 1631-1641 (2002-08-09)
By using steady state and time-resolved (laser flash photolysis and single photon counting) spectroscopic techniques the quenching of the lowest excited singlet (S1) state of 9-cyanoanthracene (9CNA) by the donors (quenchers) 2-methylindole (2MI) and 2-methylindoline (2MIN) in solvents of different
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.