Alle Fotos(1)
Wichtige Dokumente
728373
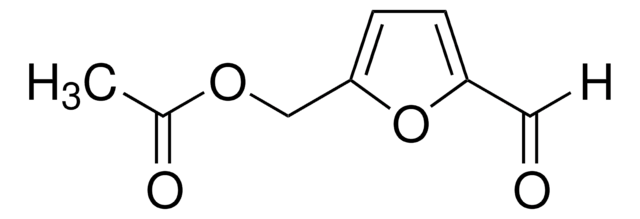
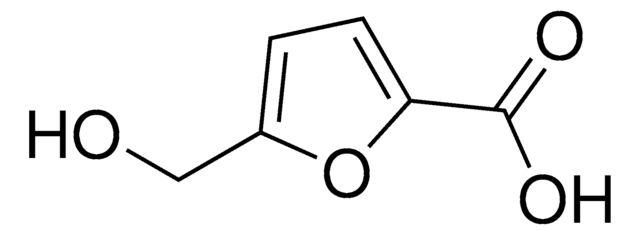
2,5-Furandicarbaldehyd
97%
Synonym(e):
2,5-Diformyl-furan, 5-Formyl-furfural
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C6H4O3
CAS-Nummer:
Molekulargewicht:
124.09
Beilstein:
109424
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥96.5% (HPLC)
97%
Form
powder
Funktionelle Gruppe
aldehyde
Lagertemp.
−20°C
SMILES String
O=Cc1ccc(C=O)o1
InChI
1S/C6H4O3/c7-3-5-1-2-6(4-8)9-5/h1-4H
InChIKey
PXJJKVNIMAZHCB-UHFFFAOYSA-N
Allgemeine Beschreibung
2,5-Furandicarboxaldehyde is an oxidation product of 5-hydroxymethyl furfural. It is used as an organic building block in chemical synthesis. It is also used as a precursor for the production of valuable biopolymers.
Anwendung
2,5- Furandicarboxaldehyde can be used in the synthesis of sustainable thin–film composite (TFC) membranes by interfacial polymerization reaction with chitosan and it also acts as a fluorescent chemo sensor for Hg2+ ions.
2,5-Furandicarboxaldehyde can be used as a building block in the fabrication of sustainable thin-film composite (TFC) membranes by the interfacial polymerization reaction with chitosan.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Solvent-resistant thin-film composite membranes from biomass-derived building blocks: chitosan and 2, 5-furandicarboxaldehyde
Park, et al.
ACS sustainable chemistry & engineering, 10, 998-1007 (2021)
Selective photocatalytic oxidation of 5-hydroxymethyl-2-furfural to 2, 5-furandicarboxyaldehyde in aqueous suspension of g-C3N4
Krivtsov, et al.
Applied Catalysis. B, Environmental, 204, 430-439 (2017)
A bis-hydrazone derivative of 2, 5-furandicarboxaldehyde with perfect hetero-atomic cavity for selective sensing of Hg (II) and its intracellular detection in living HeLa S3 cell
Kumari, et al.
Sensors and Actuators B, Chemical, 243, 1181-1190 (2017)
Kasanneni Tirumala Venkateswara Rao et al.
ChemSusChem, 11(18), 3323-3334 (2018-07-15)
A highly active and inexpensive Co-Mn mixed-oxide catalyst was prepared and used for selective oxidation of 5-hydroxymethylfurfural (HMF) into 2, 5-furandicarboxylic acid (FDCA). Co-Mn mixed-oxide catalysts with different Co/Mn molar ratios were prepared through a simple solid-state grinding method-a low-cost
Cristina Megías-Sayago et al.
Frontiers in chemistry, 8, 461-461 (2020-06-26)
A series of gold catalysts supported on pure CeO2, ZrO2, and two different Ce-Zr mixed oxides have been prepared and tested in the 5-hydroxymethyl-2-furfural oxidation reaction. All catalysts show high catalytic activity (100% conversion) and important selectivity (27-41%) to the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.



![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)