Alle Fotos(2)
Wichtige Dokumente
456764
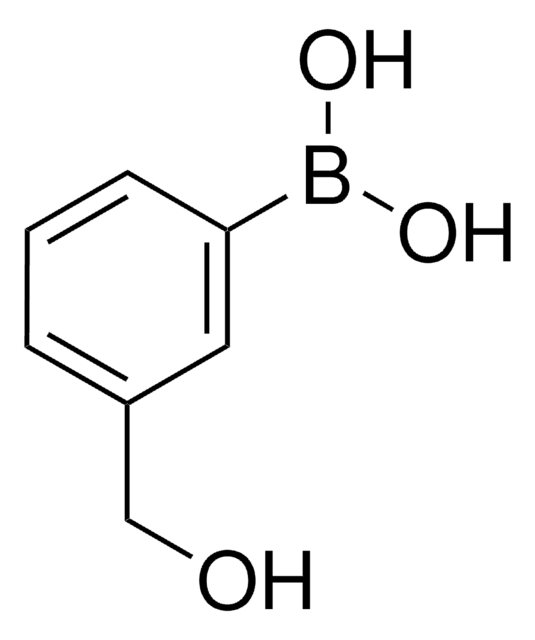
3-Carboxyphenylborsäure
≥95%
Synonym(e):
μ-Carboxyphenylboronic acid, 3-(Dihydroxyborane)benzoic acid, 3-(Dihydroxyboryl)benzoic acid, 3-Boronobenzoic acid, 3-Carboxybenzeneboronic acid
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(2)
About This Item
Lineare Formel:
HO2CC6H4B(OH)2
CAS-Nummer:
Molekulargewicht:
165.94
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥95%
mp (Schmelzpunkt)
243-247 °C (lit.)
Funktionelle Gruppe
carboxylic acid
SMILES String
OB(O)c1cccc(c1)C(O)=O
InChI
1S/C7H7BO4/c9-7(10)5-2-1-3-6(4-5)8(11)12/h1-4,11-12H,(H,9,10)
InChIKey
DBVFWZMQJQMJCB-UHFFFAOYSA-N
Anwendung
3-Carboxyphenylboronic acid can be used as a substrate in the preparation of:
- Biaryl derivatives by reacting with bromoaniline through the Suzuki-Miyaura coupling reaction.
- Boronic acid-functionalized block copolymer.
- 1H-Imidazo[1,2-a]quinoxaline derivatives.
Sonstige Hinweise
Contains varying amounts of anhydride
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 2
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Novel rhodamine dyes via Suzuki coupling of xanthone triflates with arylboroxins
Calitree, B. D.; Detty, M. R.
Synlett, 89-92 (2010)
Jumin Yang et al.
Materials science & engineering. C, Materials for biological applications, 116, 111250-111250 (2020-08-19)
Various nanoparticles as drug delivery system provide significant improvements in the cancer treatment. However, their clinical success remains elusive in large part due to their inability to overcome both systemic and tumor tissue barriers. The nanosystems with nanoproperty-transformability (surface, size
Di Wu et al.
Acta biomaterialia, 96, 123-136 (2019-06-28)
Locoregional chemotherapy, especially using implantable hydrogel depots to sustainably deliver chemotherapeutics at tumor site, has shown great potential for improving antitumor efficacy and reducing systemic toxicity. However, the hydrogel applications are limited by some intrinsic constraints, especially the contradiction between
Synthesis of a phenylboronic acid-functionalized thermosensitive block copolymer and its application in separation and purification of vicinal-diol-containing compounds
Wang Y, et al.
Royal Society of Chemistry Advances, 6(85), 82309-82320 (2016)
Christopher G Barber et al.
Bioorganic & medicinal chemistry letters, 14(12), 3227-3230 (2004-05-20)
A series of 1-isoquinolinylguanidines are shown to be potent inhibitors of uPA with selectivity over tPA and plasmin. Potency is enhanced by the presence of a 4-halo and a 7-aryl substituent, particularly when substituted by a 3-carboxylic acid group. Compound
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.