Alle Fotos(1)
Wichtige Dokumente
241636
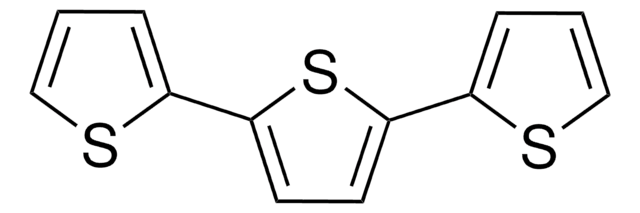
2,2′-Bithiophen
99%
Synonym(e):
2,2′-Bithienyl, 2,2′-Dithienyl
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C8H6S2
CAS-Nummer:
Molekulargewicht:
166.26
Beilstein:
3039
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352103
PubChem Substanz-ID:
NACRES:
NA.23
Empfohlene Produkte
Assay
99%
bp
260 °C (lit.)
mp (Schmelzpunkt)
32-33 °C (lit.)
SMILES String
c1csc(c1)-c2cccs2
InChI
1S/C8H6S2/c1-3-7(9-5-1)8-4-2-6-10-8/h1-6H
InChIKey
OHZAHWOAMVVGEL-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
2,2′-Bithiophene is an electron transporting material with the π-electrons present in the system that facilitate charge mobility.
Anwendung
2,2′-Bithiophene can be polymerized to form poly(2,2′-Bithiophene) which can be electrodeposited on indium tin oxide (ITO) substrates for the fabrication of electrochromic devices. It can also be used in the formation of electrode material for the development of supercapacitors.
Substrate used in a rhodium-catalyzed C-H arylation of heteroarenes with aryl iodides.
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
230.0 °F - closed cup
Flammpunkt (°C)
110 °C - closed cup
Persönliche Schutzausrüstung
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Weiying He et al.
Nature communications, 9(1), 3866-3866 (2018-09-27)
Nickel-catalyzed catalyst transfer polycondensation (CTP) of thiophenes is an efficient strategy for the controlled synthesis of polythiophenes. However, a detailed view of its reaction mechanism has remained elusive with unresolved questions regarding the geometry and bonding of critical Ni(0) thiophene intermediates.
5, 5 `-Bis (dimesitylboryl)-2, 2 `-bithiophene and 5, 5 ``-bis (dimesitylboryl)-2, 2 `: 5 `, 2 ``-terthiophene as a novel family of electron-transporting amorphous molecular materials.
Noda T and Shirota Y
Journal of the American Chemical Society, 120(37), 9714-9715 (1998)
Electrochemical characterization of the Poly (2, 2'-Bithiophene-co-Pyrene) Functionalized Single-Walled Carbon Nanotubes Films and Their Applications in Supercapacitors Field.
Baibarac M, et al.
International Journal of Electrochemical Science, 12(3), 2013-2025 (2017)
Electrode material dependent p-or n-like thermoelectric behavior of single electrochemically synthesized poly (2, 2?-bithiophene) layer?application to thin film thermoelectric generator.
Kublitski J, et al.
Journal of Solid State Electrochemistry, 20(8), 2191-2196 (2016)
Shuichi Yanagisawa et al.
Journal of the American Chemical Society, 128(36), 11748-11749 (2006-09-07)
A new method for the catalytic C-H arylation of heteroarenes and arenes that manifests high activity paired with reasonably broad scope was developed. Under the catalytic influence of RhCl(CO){P[OCH(CF3)2]3}2 and Ag2CO3, the direct C-H arylation of heteroarenes/arenes with aryl/heteroaryl iodides
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![Thieno[3,2-b]thiophen 95%](/deepweb/assets/sigmaaldrich/product/structures/353/609/429fd4bf-e217-4371-80a3-9e5a4d88908b/640/429fd4bf-e217-4371-80a3-9e5a4d88908b.png)



![2,5-Bis(trimethylstannyl)-thieno[3,2-b]thiophene 97%](/deepweb/assets/sigmaaldrich/product/structures/126/532/26557e94-858e-4c96-90de-ca88d84a8727/640/26557e94-858e-4c96-90de-ca88d84a8727.png)
![Benzo[1,2-b:4,5-b′]dithiophene-4,8-dione 97%](/deepweb/assets/sigmaaldrich/product/structures/418/544/b7faac0b-ad09-4b42-a9fa-aeb38017a39e/640/b7faac0b-ad09-4b42-a9fa-aeb38017a39e.png)