Alle Fotos(3)
Wichtige Dokumente
228435
5-Brom-nicotinsäure
98%
Synonym(e):
5-Brompyridin-3-carbonsäure
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(3)
About This Item
Empirische Formel (Hill-System):
C6H4BrNO2
CAS-Nummer:
Molekulargewicht:
202.01
Beilstein:
115854
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
98%
Form
solid
mp (Schmelzpunkt)
178-180 °C (lit.)
Funktionelle Gruppe
bromo
carboxylic acid
SMILES String
OC(=O)c1cncc(Br)c1
InChI
1S/C6H4BrNO2/c7-5-1-4(6(9)10)2-8-3-5/h1-3H,(H,9,10)
InChIKey
FQIUCPGDKPXSLL-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Anwendung
5-Bromopyridine-3-carboxylic acid (5-bromonicotinic acid) was used in the synthesis of 3-guanidinomethyl-5-iodopyridine.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Persönliche Schutzausrüstung
dust mask type N95 (US), Eyeshields, Gloves
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
G Vaidyanathan et al.
Bioconjugate chemistry, 9(6), 758-764 (1998-11-17)
Substituting a pyridine ring for a benzene ring in the acylation agent N-succinimidyl 3-iodobenzoate has resulted in a useful approach for the radiohalogenation of monoclonal antibodies, peptides, and labeled biotin conjugates. It was hypothesized that such a substitution in m-iodobenzylguanidine
F Gabor et al.
Journal of pharmaceutical sciences, 84(9), 1120-1125 (1995-09-01)
Two types of monoclonal antibodies were used for the determination of nicergoline in biological matrices. The antibodies were prepared with the hydrolysis products 5-bromonicotinic acid and 1-methyl-10 alpha-methoxydihydrolysergol after hemisuccinoylation to haptens. The current amide bond-generating methods (mixed anhydride-, carbodiimide-
Małgorzata Dukat et al.
European journal of pharmacology, 435(2-3), 171-180 (2002-02-01)
Two 5-substituted derivatives of nicotine (nicotinic acetylcholine receptor: K(i)=2.4 nM) were synthesized and evaluated: 5-bromonicotine (K(i)=6.9 nM) and 5-methoxynicotine (K(i)=14.3 nM). Despite their high affinity, neither 5-bromonicotine nor 5-methoxynicotine mimicked nicotine in producing antinociceptive (tail-flick, hotplate), hypolocomotor, or hypothermic effects
Tawfik Gharbaoui et al.
Bioorganic & medicinal chemistry letters, 17(17), 4914-4919 (2007-06-26)
A strategy for lead identification of new agonists of GPR109a, starting from known compounds shown to activate the receptor, is described. Early compound triage led to the formulation of a binding hypothesis and eventually to our focus on a series
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.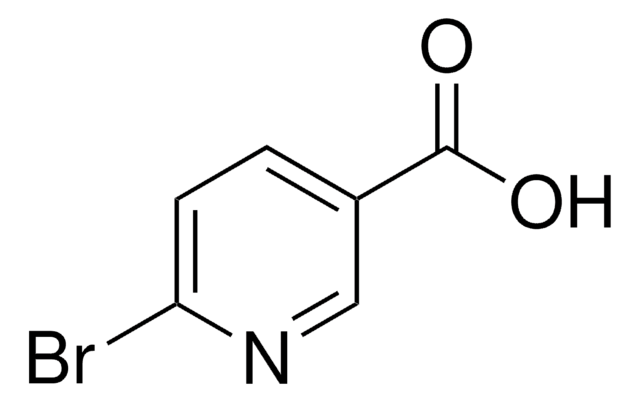



![[Pd(OAc)2]3 reagent grade, 98%](/deepweb/assets/sigmaaldrich/product/structures/508/249/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f/640/99a0ef2c-b77c-4d73-8ed9-0cca05b6b41f.png)

![[1,1′-Bis(diphenylphosphino)ferrocen]dichlorpalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

