191280
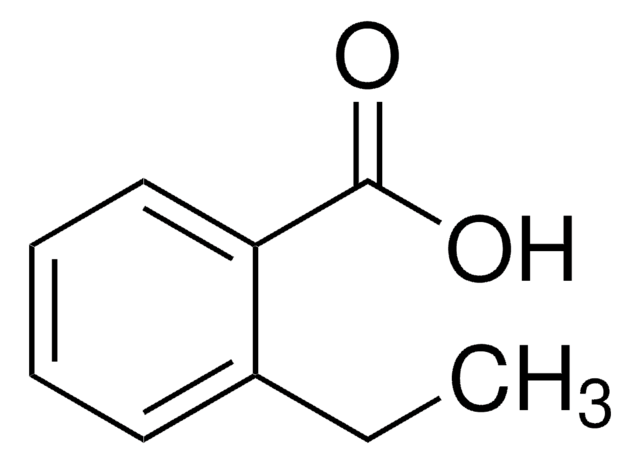
4-Ethyl-benzoesäure
99%
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
C2H5C6H4CO2H
CAS-Nummer:
Molekulargewicht:
150.17
Beilstein:
2041840
EG-Nummer:
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
99%
Form
solid
mp (Schmelzpunkt)
112-113 °C (lit.)
Funktionelle Gruppe
carboxylic acid
SMILES String
CCc1ccc(cc1)C(O)=O
InChI
1S/C9H10O2/c1-2-7-3-5-8(6-4-7)9(10)11/h3-6H,2H2,1H3,(H,10,11)
InChIKey
ZQVKTHRQIXSMGY-UHFFFAOYSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
4-Ethylbenzoic acid reacts with lanthanum nitrate in aqueous solution to yield the polymer catena-poly[[aqua(4-ethylbenzoic acid-κO)lanthanum(III)]-tri-μ-4-ethylbenzoato].
Anwendung
4-Ethylbenzoic acid was used in the synthesis of ethyl 4-vinyl-α-cyano-β-phenylcinnamate. It was also used to functionalize the edge of “pristine” graphite in the presence of polyphosphoric acid/phosphorus pentoxide.
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Hier finden Sie alle aktuellen Versionen:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Juan Yang et al.
Acta crystallographica. Section E, Structure reports online, 66(Pt 2), m183-m184 (2010-01-01)
The reaction of lanthanum nitrate and 4-ethyl-benzoic acid (EBAH) in aqueous solution yielded the title polymer, [La(C(9)H(9)O(2))(3)(C(9)H(10)O(2))(H(2)O)](n). The asymmetric unit contains one La(III) atom, three 4-ethyl-benzoate (EBA) ligands, one neutral EBAH ligand and one coordinated water mol-ecule. Each La(III) ion
Functional Polymers. VII. Ethyl 4-Vinyl-α-cyano-β-phenylcinnamate.
Sumida Y and Vogl O.
Polymer Journal, 13(6), 521-536 (1981)
José María Moreno et al.
Chemical science, 10(7), 2053-2066 (2019-03-08)
Novel MOF-type materials with different morphologies based on assembled 1D organic-inorganic sub-domains were prepared using specific monodentate benzylcarboxylate spacers with functional substituents in the para-position as structure modulating agents. The combination of electron-withdrawing or electron-donating functions in the organic spacers
J L Ramos et al.
Science (New York, N.Y.), 235(4788), 593-596 (1987-01-30)
Increasing quantities of man-made organic chemicals are released each year into the biosphere. Some of these compounds are both toxic and relatively resistant to physical, chemical, or biological degradation, and they thus constitute an environmental burden of considerable magnitude. Genetic
Ruthenium(II)-catalyzed synthesis of hydroxylated arenes with ester as an effective directing group.
Yiqing Yang et al.
Organic letters, 14(11), 2874-2877 (2012-05-16)
An unprecedented Ru(II) catalyzed ortho-hydroxylation has been developed for the facile synthesis of a variety of multifunctionalized arenes from easily accessible ethyl benzoates with ester as an efficient directing group. Both the TFA/TFAA cosolvent system and oxidants serve as the
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.